A randomised crossover trial of daridorexant for the treatment of chronic insomnia and nocturia
- PMID: 40083090
- PMCID: PMC12592811
- DOI: 10.1111/jsr.70002
A randomised crossover trial of daridorexant for the treatment of chronic insomnia and nocturia
Abstract
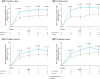
This double-blind, placebo-controlled, two-way crossover trial evaluated the efficacy and safety of daridorexant in patients with chronic insomnia and comorbid nocturia. In total, 60 patients aged ≥55 years with insomnia complaints for ≥3 months, Insomnia Severity Index (ISI) ≥13 and ≥3 voids/night for ≥1 month were randomised (1:1) to daridorexant 50 mg/placebo for 4 weeks followed by crossover after a 14-21-day washout period. The primary endpoint was change from baseline to Week (W) 4 in self-reported total sleep time (sTST). Other endpoints included change in ISI score, sleep depth and quality (visual analogue scale scores), nocturnal voids (mean number, time to first) and daytime functioning (Insomnia Daytime Symptoms and Impacts Questionnaire score [IDSIQ]). At W4, daridorexant significantly increased sTST versus placebo (least-squares mean difference [LSMD] 20.9 min, 95% confidence interval [CI] 8.0-33.7; p = 0.002); significant improvements were also seen at W1-3. Compared with placebo, daridorexant significantly decreased (p < 0.001) ISI at both timepoints, W2 (LSMD -3.7, 95% CI -5.1 to -2.3) and W4 (LSMD -3.3, 95% CI -4.7 to -1.8) and significantly improved (p < 0.05) sleep depth (W1, 2, 3, 4), sleep quality (W1, 2, 3) and IDSIQ total score (W1, 3). Daridorexant versus placebo reduced the number of voids (LSMD [95% CI]: W1-0.6 [-0.9 to -0.3], p < 0.001; W4-0.3 [-0.7 to +0.1], p = 0.090) and increased median time to first void (difference to placebo, W1: +31 min, p = 0.0027; W4: +23 min, p = 0.2026). No adverse events of special interest (falls/urinary incontinence) were reported during daridorexant treatment. In conclusion, in patients with chronic insomnia and nocturia, daridorexant improves both conditions with a favourable safety profile.
Keywords: daridorexant; dual orexin receptor antagonist; insomnia; nocturia; total sleep time; voids.
© 2025 The Author(s). Journal of Sleep Research published by John Wiley & Sons Ltd on behalf of European Sleep Research Society.
Conflict of interest statement
Heike Benes has received speaker fees from Idorsia Pharmaceuticals and participated in advisory boards from Idorsia Pharmaceuticals and Takeda. Jose Emilio Batista has received CME grants from Lacer Italfarmaco, Astellas Europe, Hollister and Coloplast. Michael Meinel, Racheal Rowles and Tobias Di Marco were all employees of Idorsia Pharmaceuticals Ltd at the time of the study. Alan Fine, David Castro Diaz, Katharina Lederer, Sandro Bacchelli, and Sylvia Shoffner have no conflicts of interest to disclose.
Figures

References
-
- Abraham, L. , Hareendran, A. , Mills, I. W. , Martin, M. L. , Abrams, P. , Drake, M. J. , MacDonagh, R. P. , & Noble, J. G. (2004). Development and validation of a quality‐of‐life measure for men with nocturia. Urology, 63, 481–486. - PubMed
-
- American Academy of Sleep Medicine . (2014). International classification of sleep disorders. American Academy of Sleep Medicine.
-
- Baglioni, C. , Altena, E. , Bjorvatn, B. , Blom, K. , Bothelius, K. , Devoto, A. , Espie, C. A. , Frase, L. , Gavriloff, D. , Tuuliki, H. , Hoflehner, A. , Högl, B. , Holzinger, B. , Järnefelt, H. , Jernelöv, S. , Johann, A. F. , Lombardo, C. , Nissen, C. , Palagini, L. , … Riemann, D. (2020). The European academy for cognitive Behavioural therapy for insomnia: An initiative of the European insomnia network to promote implementation and dissemination of treatment. Journal of Sleep Research, 29, e12967. - PubMed
-
- Bladt, L. , De Win, G. , & Wachter, S. (2022). Accuracy validation of a novel automated bladder diary device, Minze diary pod. European Urology, 81, S 151.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

