Post-exposure testing at healthcare facilities with SARS-CoV-2 transmission: A rapid review
- PMID: 40083354
- PMCID: PMC11905177
- DOI: 10.4102/jphia.v16i2.623
Post-exposure testing at healthcare facilities with SARS-CoV-2 transmission: A rapid review
Abstract
Background: Post-exposure severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing following health facility outbreaks may control the spread of infection.
Aim: This study aimed to assess the impact of testing for SARS-CoV-2 infection on health outcomes during healthcare facility outbreaks.
Setting: This review included studies conducted at skilled nursing facilities, a cancer centre, and a geriatric psychiatric facility.
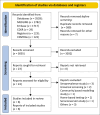
Methods: We followed the methods for conducting rapid systematic reviews, searched databases from December 2019 to August 2022, assessed the risk of bias using the modified Newcastle Ottawa scale, and graded the certainty of evidence using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) approach. We pooled the prevalence, mortality, and hospitalisation results as appropriate.
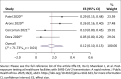
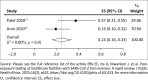
Results: Of the 3055 articles from database search, no study was eligible for inclusion as outlined in the protocol. However, eight non-comparative reports (case series) in skilled nursing facilities were included. The pooled prevalence of SARS-CoV-2 infection among residents of care homes and patients were 38% (95% confidence interval [CI] = 25% - 51%; 5 studies, 2044 participants; I 2 = 94%, very low certainty evidence) and was 12% (95% CI = 6% - 19%; 5 studies, 2312 participants; I 2 = 94%, very low certainty evidence) for exposed healthcare workers. The pooled mortality estimate and hospitalisation rate were 17% and 24%, respectively, (very low certainty evidence).
Conclusion: There is no identified evidence for or against testing of people in healthcare facilities where there is ongoing transmission of SARS-CoV-2 infection.
Contribution: The evaluation of the effectiveness of testing strategies during SARS-CoV-2 outbreaks need baseline and follow-up data from well-designed before and after studies appropriate for the setting.
Keywords: SARS-CoV-2; healthcare facilities; post-exposure testing; systematic review; transmission.
© 2025. The Authors.
Conflict of interest statement
The author reported that they received funding from Country Readiness Strengthening, WHO World Health Emergencies Programme, Geneva, Switzerland to Cochrane Nigeria, which may be affected by the research reported in the enclosed publication. The author has disclosed those interests fully and has implemented an approved plan for managing any potential conflicts arising from their involvement. The terms of these funding arrangements have been reviewed and approved by the affiliated university in accordance with its policy on objectivity in research.
Figures


References
-
- WHO . Coronavirus (COVID-19) dashboard [homepage on the Internet]. 2022. [cited 2022 Aug 24]. Available from: https://covid19.who.int/.
-
- WHO . Tracking SARS-CoV-2 variants [homepage on the Internet]. 2021. [cited 2022 Aug 24]. Available from: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
