Gut microbiota regulates optic nerve fiber myelination
- PMID: 40083662
- PMCID: PMC11904436
- DOI: 10.3389/fcell.2025.1526855
Gut microbiota regulates optic nerve fiber myelination
Abstract
Introduction: Recent evidence supports the hypothesis of an association between gut microbiota and the pathogenesis of retinal and eye diseases, suggesting the existence of a gut-eye axis. However, no data are available on the possible effect of the gut microbiota on the optic nerve fiber maturation and myelin development.
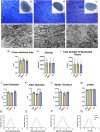
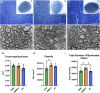
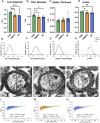
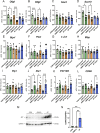
Methods: We investigated the impact of gut microbiota on the optic nerves collected from neonatal and young adult germ-free (GF), gnotobiotic (stably colonized with 12 bacteria strains, OMM12) and control (colonized with a complex gut microbiota, CGM) mice, by performing stereological and morphoquantitative analyses with transmission electron microscopy and gene expression analysis by quantitative real-time PCR.
Results: Young adult GF and OMM12 optic nerve axons are smaller and hypermyelinated compared to CGM ones, while no such differences were detected in neonatal optic nerves. The transcription factors Olig1, Olig2, and Sox10 (oligodendrocyte myelination positive regulators) are downregulated in CGM and OMM12 young adult mice compared to the respective neonates. Such developmental downregulation was not observed in GF optic nerves, suggesting that the absence of the gut microbiota prolongs the stimulation of optic nerve fiber myelination, possibly through mechanisms that are yet to be identified.
Discussion: Altogether, these data underscore the gut microbiota pivotal role in driving optic nerve myelination, contributing to our knowledge about both the gut-eye axis and the gut-brain axis, and opening new horizons for further investigations that will explore the role of the microbiota also in pathologies, injuries and regeneration associated with the optic nerve.
Keywords: germ-free mice; gnotobiotic mice; microbiota; myelin; oligodendrocytes.
Copyright © 2025 Ronchi, Pellegrino, El Soury, Amato, Gaia, Farzin, Nuzzi, Basic, Bolsega, Geuna, Cescon, Haastert-Talini and Gambarotta.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

References
LinkOut - more resources
Full Text Sources

