KAT6B overexpression in mice causes aggression, anxiety, and epilepsy
- PMID: 40083716
- PMCID: PMC11904597
- DOI: 10.1016/j.isci.2025.111953
KAT6B overexpression in mice causes aggression, anxiety, and epilepsy
Abstract
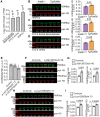
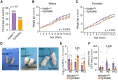
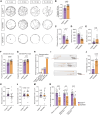
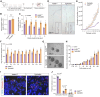
Loss of the gene encoding the histone acetyltransferase KAT6B (MYST4/MORF/QKF) causes developmental brain abnormalities as well as behavioral and cognitive defects in mice. In humans, heterozygous variants in the KAT6B gene cause two cognitive disorders, Say-Barber-Biesecker-Young-Simpson syndrome (SBBYSS; OMIM:603736) and genitopatellar syndrome (GTPTS; OMIM:606170). Although the effects of KAT6B homozygous and heterozygous mutations have been documented in humans and mice, KAT6B gain-of-function effects have not been reported. Here, we show that overexpression of the Kat6b gene in mice caused aggression, anxiety, and spontaneous epilepsy. Kat6b overexpression led to an increase in histone H3 lysine 9 acetylation and upregulation of genes driving nervous system development and neuronal differentiation. Kat6b overexpression additionally promoted neural stem cell proliferation and favored neuronal over astrocyte differentiation in vivo and in vitro. Our results suggest that, in addition to loss-of-function alleles, gain-of-function KAT6B alleles may be detrimental for brain development.
Keywords: Behavioral neuroscience; Biological sciences; Molecular neuroscience; Neuroscience.
© 2025 The Author(s).
Conflict of interest statement
A.K.V. and T.T. are inventors on patent WO2016198507A1. A.K.V. and T.T. have received research funding from the CTx CRC. A.K.V. and T.T. have served on a clinical advisory board for Pfizer.
Figures



References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

