The inflammatory endotype in osteoarthritis: Reflections from the 2024 OARSI clinical trials symposium (CTS) with a special emphasis on feasibility for clinical development
- PMID: 40083835
- PMCID: PMC11905839
- DOI: 10.1016/j.ocarto.2025.100572
The inflammatory endotype in osteoarthritis: Reflections from the 2024 OARSI clinical trials symposium (CTS) with a special emphasis on feasibility for clinical development
Abstract
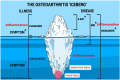
Objective: The inflammatory endotype is arguably one of the most well-established endotype in osteoarthritis (OA). While endotyping holds promise for advancing drug development, numerous potential challenges must be considered, addressed and resolved before successful clinical outcomes can be achieved.
Design: Since 2017, the Osteoarthritis Research Society International (OARSI) has hosted the Clinical Trials Symposium (CTS). Each year, OARSI and the CTS steering committee encourage discussions on selected topics among a broad range of stakeholders, including regulators, drug developers, clinicians, clinical researchers, biomarker specialists, and basic scientists, with the aim of advancing drug development in the OA field.
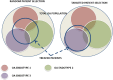
Results: This report highlights the ongoing tension between academia's "blue ocean" strategy and the feasibility-driven approach of drug developers, all within the context of scientific efforts to find effective solutions. Understanding the needs, goals, constraints, and opportunities of all involved stakeholders is crucial for defining optimal drug development strategies for OA.
Conclusion: A multidisciplinary, collaborative approach is essential for developing effective OA treatments, balancing scientific discovery with regulatory and clinical feasibility.
Keywords: Clinical development; Endotype; Inflammation; Osteoarthritis; Phenotype; Stratification.
© 2025 The Authors.
Conflict of interest statement
The authors declare no competing interests related to this work.
Figures

References
-
- A white paper by the EFPIA Clinical Trial Design Taskforce on behalf of the EFPIA Clinical Research Expert Group Innovation in clinical trial design: a review of the clinical trial design landscape. 2020 Mar 7. https://www.efpia.eu/media/547507/efpia-position-paper-innovation-in-cli...
-
- Karsdal M.A., Tambiah J., Felson D., Ladel C., Nikolov N.P., Hodgins D., et al. Reflections from the OARSI 2022 clinical trials symposium: the pain of OA-Deconstruction of pain and patient-reported outcome measures for the benefit of patients and clinical trial design. Osteoarthr. Cartil.[Internet] 2023 Jun 26 http://www.ncbi.nlm.nih.gov/pubmed/37380011 Available from: - PMC - PubMed
-
- Karsdal M.A., Tambiah J., Hochberg M.C., Ladel C., Bay-Jensen A.C., Arendt-Nielsen L., et al. Reflections from the 2021 OARSI clinical trial symposium: considerations for understanding biomarker assessments in osteoarthritis drug development - should future studies focus on disease activity, rather than status? Osteoarthr. Cartil. Open [Internet] 2022 Sep;4(3) https://linkinghub.elsevier.com/retrieve/pii/S2665913122000309 Available from: - PMC - PubMed
-
- Eichler H.G., Pignatti F., Flamion B., Leufkens H., Breckenridge A. Balancing early market access to new drugs with the need for benefit/risk data: a mounting dilemma. Nat. Rev. Drug Discov. 2008 Oct 12;7(10):818–826. - PubMed
-
- Dugger S.A., Platt A., Goldstein D.B. Drug development in the era of precision medicine. Nat Rev Drug Discov [Internet] 2018;17(3):183–196. http://www.ncbi.nlm.nih.gov/pubmed/29217837 Available from: - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

