Targeting platinum-resistant ovarian cancer by disrupting histone and RAD51 lactylation
- PMID: 40083924
- PMCID: PMC11898288
- DOI: 10.7150/thno.104858
Targeting platinum-resistant ovarian cancer by disrupting histone and RAD51 lactylation
Abstract
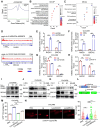
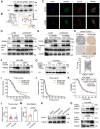
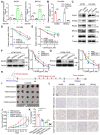
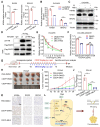
Rationale: Ovarian cancer is a highly lethal gynecological malignancy with common platinum resistance. Lactylation is involved in multiple biological processes. Thus, we explored the role of histone and non-histone lactylation in platinum resistance, providing a potential therapeutic target to overcome platinum resistance in ovarian cancer. Methods: We utilized gene set enrichment analysis to investigate lactylation-related pathway alterations between platinum-resistant and platinum-sensitive patients from the TCGA cohort. Differential expression of H3K9la was demonstrated using Western blotting and immunohistochemistry. Progression-free and overall survival were determined using a log-rank test. Drug response to cisplatin was evaluated by CCK8, apoptosis flow cytometry, and clonogenic assays in vitro. ChIP-seq and ChIP-qPCR assays were performed to identify downstream targets of H3K9la, which was further confirmed by qRT-PCR. LC-MS/MS was conducted to identify specific lactylation sites for RAD51. Co-IP was used to reveal the interaction between GCN5 and H3K9la or RAD51la. Cell line-derived and patient-derived xenograft (PDX) models of ovarian cancer were constructed for the in vivo experiments. Results: Our study showed elevated histone lactylation, especially of H3K9la, in platinum-resistant ovarian cancer. Moreover, high H3K9la indicated platinum resistance and poor prognosis of ovarian cancer. Impairing H3K9la enhanced response to cisplatin. Mechanistically, H3K9la directly activated RAD51 and BRCA2 expression to facilitate homologous recombination (HR) repair. Furthermore, RAD51K73la enhanced HR repair and subsequently conferred cisplatin resistance. H3K9la and RAD51K73la shared the same upstream regulator, GCN5. Notably, a GCN5 inhibitor remarkably improved the tumor-killing ability of cisplatin in PDX models of ovarian cancer. Conclusions: Our study demonstrated the essential role of histone and RAD51 lactylation in HR repair and platinum resistance. It also identified a potential therapeutic strategy to overcome platinum resistance and improve prognosis in ovarian cancer.
Keywords: HR repair; RAD51; lactylation; ovarian cancer; platinum resistance.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. - PubMed
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49. - PubMed
-
- Richardson DL, Eskander RN, O'Malley DM. Advances in Ovarian Cancer Care and Unmet Treatment Needs for Patients With Platinum Resistance: A Narrative Review. JAMA Oncol. 2023;9:851–9. - PubMed
-
- Lheureux S, Gourley C, Vergote I, Oza AM. Epithelial ovarian cancer. Lancet. 2019;393:1240–53. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

