Hippocampal P2X7 and A2A purinoceptors mediate cognitive impairment caused by long-lasting epileptic seizures
- PMID: 40083937
- PMCID: PMC11898279
- DOI: 10.7150/thno.100365
Hippocampal P2X7 and A2A purinoceptors mediate cognitive impairment caused by long-lasting epileptic seizures
Abstract
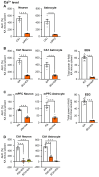
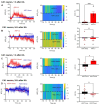
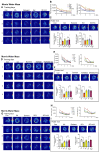
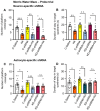
Rationale: Cognitive impairment and depression are salient comorbidities of mesial temporal lobe epilepsy; it is still unclear whether this frequently drug resistant disease is a cause or consequence of hippocampal damage and its interplay with long-lasting seizure activity (status epilepticus; SE). Thus, a major therapeutic advance in this field is badly needed. Methods: We modeled enduring behavioral and electroencephalographic (EEG) seizures in mice by the intraperitoneal injection of kainic acid (KA), and measured the dynamics of the intracellular Ca2+ signals in the hippocampal CA1 area by fiber photometry. Learning and memory were controlled by the Morris Water-Maze and Novel Object Recognition tests on whole animals and by the induction of long-term potentiation in CA1 pyramidal neurons in brain slices. Depressive-like reactions were evaluated by the Tail Suspension, Forced Swim, and Sucrose Preference tests. Results: The intraperitoneal injection of the blood-brain permeable, highly selective, P2X7 and A2A receptor (R) antagonists, JNJ-47965567, and KW6002/SCH58261, respectively, counteracted the effects of KA-induced SE both on seizure activity and the increase of Ca2+ signals (as a measure of changes in the intracellular Ca2+ concentration) in neurons and astrocytes of the hippocampal CA1 area. In addition, these drugs also prevented the impairment of the hippocampus-dependent spatial and non-spatial learning abilities by KA-SE. The knockdown of P2X7Rs in CA1 astrocytes, but not neurons prevented the cognitive deterioration, suggesting that the release of astrocytic signaling molecules onto neighboring neurons might be the cause of this effect. In accordance with our observations, in hippocampal slices prepared from mice which underwent KA-SE, a selective sensitivity increases to the prototypic P2X7R agonist dibenzoyl-ATP (Bz-ATP) manifested in CA1 neurons. This sensitivity increase appeared to be due to a postsynaptic interference between P2X7Rs and the release of excitatory neurotransmitters during SE. In spite of a P2X7 and A2AR-mediated increase of Ca2+ signaling in the medial prefrontal cortex, no similar change was noted after KA-SE in depressive-like reactions or the open-field behavior. Conclusions: SE induced the release of ATP and adenosine from the hippocampus and in consequence decreased the cognitive abilities of mice. The pharmacological blockade of P2X7 and A2ARs prevented the SE-induced seizure activity and cognitive deterioration, but not depressive-like behavior.
Keywords: A2A receptors; ATP; P2X7 receptors; adenosine; hippocampus; pharmacological antagonists; status epilepticus.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





References
-
- Moshé SL, Perucca E, Ryvlin P, Tomson T. Epilepsy: new advances. Lancet. 2015;385(9971):884–98. - PubMed
-
- Jefferys JG. Basic mechanisms of focal epilepsies. Exp Physiol. 1990;75(2):127–62. - PubMed
-
- Perucca P, Bahlo M, Berkovic SF. The Genetics of Epilepsy. Annu Rev Genomics Hum Genet. 2020;21:205–30. - PubMed
-
- Walker MC. Pathophysiology of status epilepticus. Neurosci Lett. 2018;667:84–91. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

