Pathophysiology and therapies of CKD-associated secondary hyperparathyroidism
- PMID: 40083954
- PMCID: PMC11903092
- DOI: 10.1093/ckj/sfae423
Pathophysiology and therapies of CKD-associated secondary hyperparathyroidism
Abstract
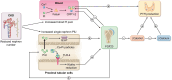
Uremic secondary hyperparathyroidism (SHP) refers to the biochemical abnormalities that characterize CKD-MBD. However, historically parathyroid hormone (PTH) is identified as the key culprit hormone and the essential biomarker of secondary hyperparathyroidism. SHP represents the adaptive response to several mineral abnormalities that initiate and maintain increased PTH secretion through classical mineral derangements and more recently elucidated hormonal dysregulations. Among classic factors involved in the pathogenesis of SHP, phosphate, calcium, and calcitriol have a prominent role. The discovery of new pathogenetic factors involved in the development of SHP (and the eventual CKD-MBD) including fibroblast growth factor-23 (FGF23) and klotho provides new hypothesis and perspectives to our understanding of this complex metabolic disturbance. Recently more than serum phosphate a critical role in regulating FGF23 synthesis and the progression of CKD is ascribed to phosphate pool, reflected by production of glycerol-3-phosphate and the formation of excessive CPP-2. Finally, also skeletal resistance to PTH action, due to dysregulation of the Wnt-β-catenin system and intestinal dysbiosis, affecting the PTH actions on bone are causal factor of SHP. Identifying all the actors at play is mandatory to allow the most precise therapeutic prescription in the individual patient. This paper aims to review, in particular, the pathophysiology of SHP, which is essential to envisage the eventual therapeutic options for the associated MBD.
Keywords: CKD-MBD; FGF23; Phosphate pool; Secondary Hyperparathyroidsm (SHP); glycerol-3-phosphate (G3P); intestinal dysbiosis.
© The Author(s) 2025. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
S.M. declares he is board member of the Società Italiana Nefrologia; L.T. has nothing to disclose; M.C.S. declares receipt of honoraria from Kyowa Kirin International and travel support from Kyowa Kirin International, all unrelated to the submitted work; M.H.T. has nothing to disclose; M.P. has nothing to disclose; S.R. has nothing to disclose; P.U.T. declares receipt of honoraria from Amgen and Theradial, consulting fees from Astra Zeneka, GSK, and Medici, and travel support from Astra Zenaka, Hemotech, and Theradial, all unrelated to the submitted work
Figures
References
-
- Moe S, Drüeke T, Cunningham J et al. Kidney Disease: Improving Global Outcomes (KDIGO). Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2006;69:1945–53. - PubMed
-
- KDIGO: KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl: 2009;S1-130. - PubMed
-
- Covic A, Vervloet M, Massy ZA et al. Bone and mineral disorders in chronic kidney disease: implications for cardiovascular health and ageing in the general population. Lancet Diabetes Endocrinol 2017;. - PubMed
LinkOut - more resources
Full Text Sources

