Selecting patients with sickle cell disease for gene addition or gene editing-based therapeutic approaches: Report on behalf of a joint EHA Specialized Working Group and EBMT Hemoglobinopathies Working Party consensus conference
- PMID: 40084235
- PMCID: PMC11904809
- DOI: 10.1002/hem3.70089
Selecting patients with sickle cell disease for gene addition or gene editing-based therapeutic approaches: Report on behalf of a joint EHA Specialized Working Group and EBMT Hemoglobinopathies Working Party consensus conference
Abstract
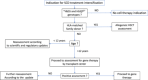
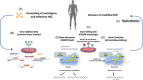
Sickle cell disease (SCD) remains associated with reduced life expectancy and poor quality of life despite improvements observed in the last decades mostly related to comprehensive care, use of hydroxycarbamide, screening to identify patients at risk of strokes, and implementation of safe transfusion protocols. The course of the disease is highly variable, making it difficult to predict severity and response to therapy. Allogeneic hematopoietic stem cell transplantation potentially provides a cure with a relatively low rate of complications, but few patients have an HLA-identical sibling. The hopes of patients and healthcare providers have been raised after the initial excellent results of gene therapy studies. However, there is a strong contrast between the high expectations of families and patients and the limited availability of the product, which is technically complex and very expensive. In light of this consideration and of the limited data available on the long-term efficacy and toxicity of different gene therapy approaches, the European Hematology Association Red Cell & Iron Specialized Working Group (EHA SWG) and the hemoglobinopathy working part of the European Blood & Marrow Transplant (EBMT) Group have prioritized the development of recommendations for selection of patients with SCD who are good candidates for gene therapy. The decision-making algorithm was developed by a panel of experts in hemoglobinopathies and/or transplantation chosen by EHA SWG and EBMT, to discuss the selection of SCD patients for gene therapy and draw notes on the related clinical problems.
© 2025 The Author(s). HemaSphere published by John Wiley & Sons Ltd on behalf of European Hematology Association.
Conflict of interest statement
Lucia de Franceschi: Agios and Bristol research grants, Roche consultant. Franco Locatelli: Speaker's bureau for Amgen, Miltenyi, Novartis, BMS, Gilead, SOBI and served on an advisory board for Amgen, Novartis, Sanofi and Vertex Pharmaceuticals Incorporated. Stefano Rivella: Scientific advisory board member of Ionis Pharmaceuticals, Meira GTx, Vifor, and Disc Medicine. Present‐last 5 years: Stefano Rivella has been or is a consultant for GSK, BMS, Incyte, Cambridge Healthcare Res, Celgene Corporation, Catenion, First Manhattan Co., FORMA Therapeutics, Ghost Tree Capital, Keros Therapeutics, Noble insight, Protagonist Therapeutics, Sanofi Aventis U.S., Slingshot Insight, Spexis AG, Techspert.io, BVF Partners L.P., Rallybio, LLC, venBio Select LLC, ExpertConnect LLC, LifeSci Capital. Stephan Lobitz: Consultant—Novartis, Vertex, Agios, Global blood therapeutics; Grant/contract: Novartis; Data and safety monitoring: Vertex. Miguel R. Abboud: Vertex: Data monitoring committee; Pfizer: Research funding; Emmaus: Speaker honoraria; Novo Nordisk: Research funding and preceptorship speaker; Novartis: Research funding; Roche: Travel support; Agios: research funding and Advisory board Josu de la Fuente: Steering Committee: Sanofi, VERTEX, Sangamo; Advisory Board: bluebird bio, VERTEX, Novartis, BEAM Therapeutics, Jazz, Roche, MAAT Pharma; Speaker Fees: Jazz, VERTEX; Research Grant: bluebird bio. Emanuele Angelucci: Data Monitoring Committee chair for Vertex and BMS, DMC member for Vifor; Consultant for Menarini‐Stemline and Sanofi; Participation in advisory board for Regeneron. Received travel support from AbbVie, Sanofi, and GILEAD. Mariane de Montalembert: Vertex, Theravia, Novartis, Vifor Boards.
Figures

References
-
- Iqbal M, Reljic T, Corbacioglu S, et al. Systematic review/meta‐analysis on efficacy of allogeneic hematopoietic cell transplantation in sickle cell disease: an international effort on behalf of the Pediatric Diseases Working Party of European Society for Blood and Marrow Transplantation and the Sickle Cell Transplantation International Consortium. Transplant Cell Ther. 2021;27:167. 10.1016/j.jtct.2020.10.007 - DOI - PubMed
-
- Mentzer WC, Heller S, Pearle PR, Hackney E, Vichinsky E. Availability of related donors for bone marrow transplantation in sickle cell anemia. Am J Pediatr Hematol Oncol. 1994;16:27‐29. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
