Neurostimulation devices to treat Alzheimer's disease
- PMID: 40084342
- PMCID: PMC11904933
- DOI: 10.37349/en.2025.100674
Neurostimulation devices to treat Alzheimer's disease
Abstract
The use of neurostimulation devices for the treatment of Alzheimer's disease (AD) is a growing field. In this review, we examine the mechanism of action and therapeutic indications of these neurostimulation devices in the AD process. Rapid advancements in neurostimulation technologies are providing non-pharmacological relief to patients affected by AD pathology. Neurostimulation therapies include electrical stimulation that targets the circuitry-level connection in important brain areas such as the hippocampus to induce therapeutic neuromodulation of dysfunctional neural circuitry and electromagnetic field (EMF) stimulation that targets anti-amyloid molecular pathways to promote the degradation of beta-amyloid (Aβ). These devices target specific or diffuse cortical and subcortical brain areas to modulate neuronal activity at the electrophysiological or molecular pathway level, providing therapeutic effects for AD. This review attempts to determine the most effective and safe neurostimulation device for AD and provides an overview of potential and current clinical indications. Several EMF devices have shown a beneficial or harmful effect in cell cultures and animal models but not in AD human studies. These contradictory results may be related to the stimulation parameters of these devices, such as frequency, penetration depth, power deposition measured by specific absorption rate, time of exposure, type of cell, and tissue dielectric properties. Based on this, determining the optimal stimulation parameters for EMF devices in AD and understanding their mechanism of action is essential to promote their clinical application, our review suggests that repeated EMF stimulation (REMFS) is the most appropriate device for human AD treatments. Before its clinical application, it is necessary to consider the complicated and interconnected genetic and epigenetic effects of REMFS-biological system interaction. This will move forward the urgently needed therapy of EMF in human AD.
Keywords: Alzheimer’s disease; clinical; devices; electromagnetic fields stimulation; preclinical; review; treatment.
Conflict of interest statement
Conflicts of interest Figure 2 was modified the image from a patent application “Electromagnetic Frequency Generation System and Method”. The patent number is pending. The patent is of no financial interest to the subject matter and material of the manuscript.
Figures

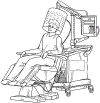
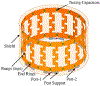
References
Grants and funding
LinkOut - more resources
Full Text Sources
