Coacervates as enzymatic microreactors
- PMID: 40084439
- PMCID: PMC11907334
- DOI: 10.1039/d4cs01203h
Coacervates as enzymatic microreactors
Abstract
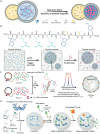
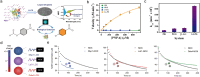
Compartmentalization, a key aspect of biochemical regulation, naturally occurs in cellular organelles, including biomolecular condensates formed through liquid-liquid phase separation (LLPS). Inspired by biological compartments, synthetic coacervates have emerged as versatile microreactors, which can provide customed environments for enzymatic reactions. In this review, we explore recent advances in coacervate-based microreactors, while emphasizing the mechanisms by which coacervates accelerate enzymatic reactions, namely by enhancing substrate and enzyme concentrations, stabilizing intermediates, and providing molecular crowding. We discuss diverse coacervate systems, including those based on synthetic polymers, peptides, and nucleic acids, and describe the selection of enzymatic model systems, as well as strategies for enzyme recruitment and their impact on reaction kinetics. Furthermore, we discuss the challenges in monitoring reactions within coacervates and review the currently available techniques including fluorescence techniques, chromatography, and NMR spectroscopy. Altogether, this review offers a comprehensive perspective on recent progress and challenges in the design of coacervate microreactors, and addresses their potential in biocatalysis, synthetic biology, and nanotechnology.
Conflict of interest statement
There are no conflicts to declare.
Figures







References
-
- Uversky V. N., Droplets of Life, Elsevier, 2023, pp. 101–132
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

