Cell-free DNA aneuploidy score as a dynamic early response marker in prostate cancer
- PMID: 40084488
- PMCID: PMC12515704
- DOI: 10.1002/1878-0261.13797
Cell-free DNA aneuploidy score as a dynamic early response marker in prostate cancer
Abstract
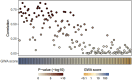
Cell-free circulating tumor DNA (ctDNA) has emerged as a promising biomarker for response evaluation in metastatic castration-resistant prostate cancer (mCRPC). The current study evaluated the modified fast aneuploidy screening test-sequencing system (mFast-SeqS), a quick, tumor-agnostic and affordable ctDNA assay that requires a small input of DNA, to generate a genome-wide aneuploidy (GWA) score in mCRPC patients, and correlated this to matched metastatic tumor biopsies. In this prospective multicenter study, GWA scores were evaluated from blood samples of 196 mCRPC patients prior to treatment (baseline) with taxanes (docetaxel and cabazitaxel) and androgen receptor signaling inhibitors (ARSI; abiraterone and enzalutamide), and from 74 mCRPC patients at an early timepoint during treatment (early timepoint; median 21 days). Z-scores per chromosome arm were tested for their association with tumor tissue genomic alterations. We found that a high tumor load in blood (GWAhigh) at baseline was associated with poor response to ARSI [HR: 2.63 (95% CI: 1.86-3.72) P < 0.001] but not to taxanes. Interestingly, GWAhigh score at the early timepoint was associated with poor response to both ARSIs [HR: 6.73 (95% CI: 2.60-17.42) P < 0.001] and taxanes [2.79 (95% CI: 1.34-5.78) P = 0.006]. A significant interaction in Cox proportional hazards analyses was seen when combining GWA status and type of treatment (at baseline P = 0.008; early timepoint P = 0.018). In summary, detection of ctDNA in blood by mFast-SeqS is cheap, fast and feasible, and could be used at different timepoints as a potential predictor for outcome to ARSI and taxane treatment in mCRPC.
Keywords: abiraterone; cabazitaxel; ctDNA; docetaxel; enzalutamide; prostate cancer.
© 2025 The Author(s). Molecular Oncology published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
JWMM declares a consultancy fee by Novartis, and institutional research grants by Pfizer and GSK (all outside the submitted work). PH has received consulting or advisory fees from Astellas, MSD, Pfizer AstraZeneca, BMS and Ipsen. MPL received institutional grants from JnJ, Astellas, MSD and Sanofi, consulting fees from Sanofi, Johnson & Johnson, Merck, Astellas, Incyte, Amgen, Janssen Cilag, Bayer, Servier and Pfizer, and is currently employed by Amgen. RW has advisory or speakers fees for Astellas, Merck, Bayer and received institutional research grants from Bayer. MSH has received institutional research funding from Bristol Myers Squibb, 4SC, Roche, Astellas Pharma Netherlands and AstraZeneca, and institutional consultancy fees from Bristol‐Myers Squibb, Roche, Merck Sharp & Dohme, AstraZeneca, Pfizer, Janssen, and Seattle Genetics. The remaining authors declare no conflict of interest.
Figures


References
-
- Annala M, Fu S, Bacon JVW, Sipola J, Iqbal N, Ferrario C, et al. Cabazitaxel versus abiraterone or enzalutamide in poor prognosis metastatic castration‐resistant prostate cancer: a multicentre, randomised, open‐label, phase II trial. Ann Oncol. 2021;32:896–905. - PubMed
-
- Evans CP, Higano CS, Keane T, Andriole G, Saad F, Iversen P, et al. The PREVAIL study: primary outcomes by site and extent of baseline disease for enzalutamide‐treated men with chemotherapy‐naive metastatic castration‐resistant prostate cancer. Eur Urol. 2016;70:675–683. 10.1016/j.eururo.2016.03.017 - DOI - PubMed
-
- Miller K, Carles J, Gschwend JE, Van Poppel H, Diels J, Brookman‐May SD. The phase 3 COU‐AA‐302 study of abiraterone acetate plus prednisone in men with chemotherapy‐naïve metastatic castration‐resistant prostate cancer: stratified analysis based on pain, prostate‐specific antigen, and Gleason score. Eur Urol. 2018;74:17–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

