Dystrophin isoform deficiency and upper-limb and respiratory function in Duchenne muscular dystrophy
- PMID: 40084496
- PMCID: PMC12426305
- DOI: 10.1111/dmcn.16282
Dystrophin isoform deficiency and upper-limb and respiratory function in Duchenne muscular dystrophy
Abstract
Aim: To investigate the associations between mutations expected to differentially affect Dp140 expression and long-term trajectories of respiratory and upper-limb motor outcomes in Duchenne muscular dystrophy (DMD).
Method: In a retrospective analysis of population-based longitudinal data from three real-world and natural history data sources, individuals with DMD aged 5 years to 18 years were subdivided according to the predicted effects of the participants' DMD mutation on dystrophin isoform expression (group 1, Dp427 absent, Dp140/Dp71 present; group 2, Dp427/Dp140 absent, Dp71 present).
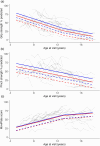
Results: A total of 459 participants were studied, with upper-limb outcomes assessed in 71 (27 in group 1 and 44 in group 2) and forced vital capacity percentage predicted (%pred) assessed in 434 (224 in group 1 and 210 in group 2). Mean grip strength %pred was on average 7.1 percentage points lower in group 2 than in group 1 (p = 0.03). Mean pinch strength %pred was on average 9.2 percentage points lower in group 2 than in group 1 (p = 0.04). Mean forced vital capacity %pred was on average 4.3 percentage points lower in group 2 than in group 1 (p = 0.01).
Interpretation: In individuals with DMD, DMD mutations predicted to affect Dp140 expression were associated with more severe trajectories of respiratory and upper-limb motor outcomes.
© 2025 The Author(s). Developmental Medicine & Child Neurology published by John Wiley & Sons Ltd on behalf of Mac Keith Press.
Conflict of interest statement
Mary Chesshyre, Deborah Ridout, Georgia Stimpson, Silvana De Lucia, and Adnan Manzur report no conflicts of interest.
Erik Niks has been a participant in advisory boards for Edgewise, Italfarmaco, Sarepta Therapeutics, Epirium, Regenxbio, and Janssen. Reimbursements were received by the Leiden University Medical Center. He has also worked as Principal Investigator at the Leiden University Medical Center for clinical trials related to muscular dystrophies from Edgewise, Italfarmaco, Sarepta Therapeutics, Fibrogen, NS Pharma, Reveragen, Santhera, BioMarin, and ML Bio.
Jean‐Yves Hogrel is a coinventor of the MyoGrip, MyoPinch, and MoviPlate devices.
Laurent Servais has received consulting fees from Roche, Biogen, Avexis, Cytokinetics, Sarepta Therapeutics, Biomarin, Pfizer, Santhera, Servier, Biophytis, Audentes, Affinia, BioHaven, Scholar Rock, Dyne, Sysnav, PTC, and Dynacure. He conducts research (newborn screening) funded by Roche, Novartis and Biogen. He is a coinventor of the MoviPlate device.
Volker Straub has served on advisory boards for Astellas Gene Therapies, Biogen, Edgewise Therapeutics, Ipsen, Kate Therapeutics, ML Bio Solutions, Novartis Gene Therapies, PepGen, Roche, Sanofi, Sarepta Therapeutics, Vertex Pharmaceuticals, and Wave Therapeutics. He has received speaking fees or honoraria from Novartis Gene Therapies, Pfizer, Roche, Sanofi, and Sarepta Therapeutics; he has received grants for clinical research from Sarepta Therapeutics and Sanofi.
Valeria Ricotti is cofounder of DiNAQOR, Parterra Limited, Salanar Limited, and Vesalic Limited; in the past, she acted as consultant for Solid Bioscience and Antisense Therapeutics.
Giovanni Baranello is Principal Investigator of clinical trials sponsored by Roche, Novartis, Sarepta Therapeutics, Pfizer, NS Pharma, Reveragen, Percheron, Biomarin, and Scholar Rock; he has received speaker or consulting fees from Sarepta Therapeutics, PTC Therapeutics, Pfizer, Biogen, Novartis Gene Therapies (AveXis), and Roche, and grants from Sarepta Therapeutics, Roche, and Novartis Gene Therapies. UCL has received funding from Sarepta Therapeutics, Roche, Pfizer, Italfarmaco, and Santhera.
Francesco Muntoni reports research funding from Sarepta Therapeutics, and participation in scientific advisory boards or clinical trial monitoring groups for Sarepta Therapeutics, Dyne Therapeutics, Dyne, Pfizer, Italfarmaco, and Santhera.
Figures

References
-
- Birnkrant DJ, Bushby K, Bann CM, Apkon SD, Blackwell A, Brumbaugh D, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol. 2018. Mar;17(3):251–67. - PMC - PubMed
-
- Darras BT, Menache‐Starobinski CC, Hinton V, Kunkel LM. Chapter 30 ‐ Dystrophinopathies. In: Darras BT, Jones HR, Ryan MM, De Vivo Childhood, and Adolescence (Second Edition) DCBTND of I, editors. San Diego: Academic Press; 2015. p. 551–92.
-
- Doorenweerd N. Combining genetics, neuropsychology and neuroimaging to improve understanding of brain involvement in Duchenne muscular dystrophy ‐ a narrative review. Neuromuscul Disord. 2020. Jun;30(6):437–42. - PubMed
-
- Pane M, Scalise R, Berardinelli A, D'Angelo G, Ricotti V, Alfieri P, et al. Early neurodevelopmental assessment in Duchenne muscular dystrophy. Neuromuscul Disord. 2013. Jun;23(6):451–5. - PubMed
-
- Ricotti V, Mandy WPL, Scoto M, Pane M, Deconinck N, Messina S, et al. Neurodevelopmental, emotional, and behavioural problems in Duchenne muscular dystrophy in relation to underlying dystrophin gene mutations. Dev Med Child Neurol. 2016. Jan;58(1):77–84. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

