Assessing Mucosal Recovery During the First 15 Months of Adopting a Gluten-Free Diet in Children With Celiac Disease
- PMID: 40084739
- PMCID: PMC12208375
- DOI: 10.14309/ajg.0000000000003400
Assessing Mucosal Recovery During the First 15 Months of Adopting a Gluten-Free Diet in Children With Celiac Disease
Abstract
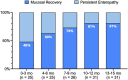
Introduction: Determine contemporary rates of early mucosal recovery in children with celiac disease (CeD).
Methods: Multicenter retrospective cohort study in Canada and the United States. Children diagnosed with CeD between 2016 and 2021 who underwent a follow-up biopsy within 15 months of diagnosis were included.
Results: Overall, 96 of 130 children (74%) had mucosal recovery, including 48% children (12/25) who were assessed within 3 months. Musculoskeletal symptoms at diagnosis were the only clinical characteristic associated with persistent enteropathy.
Discussion: While mucosal recovery can occur within months, more than a quarter of children with CeD had persistent enteropathy within 15 months of treatment.
Keywords: celiac disease; enteropathy; gluten-free diet; mucosal recovery; pediatric.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of The American College of Gastroenterology.
Conflict of interest statement
Figures
References
-
- Mearin ML, Agardh D, Antunes H, et al. ESPGHAN position paper on management and follow-up of children and adolescents with celiac disease. J Pediatr Gastroenterol Nutr 2022;75(3):369–86. - PubMed
-
- Ghazzawi Y, Rubio-Tapia A, Murray JA, et al. Mucosal healing in children with treated celiac disease. J Pediatr Gastroenterol Nutr 2014;59(2):229–31. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical