Blocking ITGA5 potentiates the efficacy of anti-PD-1 therapy on glioblastoma by remodeling tumor-associated macrophages
- PMID: 40084746
- PMCID: PMC12187582
- DOI: 10.1002/cac2.70016
Blocking ITGA5 potentiates the efficacy of anti-PD-1 therapy on glioblastoma by remodeling tumor-associated macrophages
Abstract
Background: Glioblastoma (GBM) is largely refractory to antibodies against programmed cell death 1 (anti-PD-1) therapy. Fully understanding the cellular heterogeneity and immune adaptations in response to anti-PD-1 therapy is necessary to design more effective immunotherapies for GBM. This study aimed to dissect the molecular mechanisms of specific immunosuppressive subpopulations to drive anti-PD-1 resistance in GBM.
Methods: We systematically analysed single-cell RNA sequencing and spatial transcriptomics data from GBM tissues receiving anti-PD-1 therapy to characterize the microenvironment alterations. The biological functions of a novel circular RNA (circRNA) were validated both in vitro and in vivo. Mechanically, co-immunoprecipitation, RNA immunoprecipitation and pull-down assays were conducted.
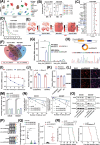
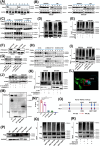
Results: Mesenchymal GBM (MES-GBM) cells, which were associated with a poor prognosis, and secreted phosphoprotein 1 (SPP1)+ myeloid-derived macrophages (SPP1+ MDMs), a unique subpopulation of MDMs with complex functions, preferentially accumulated in non-responders to anti-PD-1 therapy, indicating that MES-GBM cells and SPP1+ MDMs were the main anti-PD-1-resistant cell subpopulations. Functionally, we determined that circular RNA succinate dehydrogenase complex assembly factor 2 (circSDHAF2), which was positively associated with the abundance of these two anti-PD-1-resistant cell subpopulations, facilitated the formation of a regional MES-GBM and SPP1+ MDM cell interaction loop, resulting in a spatially specific adaptive immunosuppressive microenvironment. Mechanically, we found that circSDHAF2 promoted MES-GBM cell formation by stabilizing the integrin alpha 5 (ITGA5) protein through N-glycosylation. Meanwhile, the N-glycosylation of the ITGA5 protein facilitated its translocation into exosomes and subsequent delivery to MDMs to induce the formation of SPP1+ MDMs, which in turn maintained the MES-GBM cell status and induced T-cell dysfunction via the SPP1-ITGA5 pathway, ultimately promoting GBM immune escape. Importantly, our findings demonstrated that antibody-mediated ITGA5 blockade enhanced anti-PD-1-mediated antitumor immunity.
Conclusions: This work elucidated the potential tissue adaptation mechanism of intratumoral dynamic interactions between MES-GBM cells, MDMs and T cells in anti-PD-1 non-responders and identified the therapeutic potential of targeting ITGA5 to reduce anti-PD-1 resistance in GBM.
Keywords: Anti‐PD‐1 therapy; N‐glycosylation; exosomes; glioblastoma; intergrins; tumor‐associated macrophages.
© 2025 The Author(s). Cancer Communications published by John Wiley & Sons Australia, Ltd. on behalf of Sun Yat‐sen University Cancer Center.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- McKinnon C, Nandhabalan M, Murray SA, Plaha P. Glioblastoma: clinical presentation, diagnosis, and management. BMJ. 2021;374:n1560. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

