Impact of tebipenem pivoxil on the intestinal microbiota and on establishment of colonization with carbapenem-resistant Klebsiella pneumoniae in mice
- PMID: 40084911
- PMCID: PMC12054177
- DOI: 10.1128/spectrum.02346-24
Impact of tebipenem pivoxil on the intestinal microbiota and on establishment of colonization with carbapenem-resistant Klebsiella pneumoniae in mice
Abstract
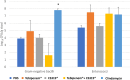
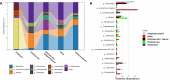
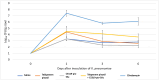
Tebipenem pivoxil has potent in vitro activity against Enterobacterales pathogens, but requires combination with β-lactamase inhibitor to achieve activity against carbapenemase producers, including metallo-β-lactamases (MBLs). Herein, we evaluate the potential of tebipenem pivoxil, alone and in combination with the prodrug of the experimental MBL inhibitor CS319 (CS319-piv-SAc), to disrupt the indigenous mice microbiota of the colon and promote colonization by pathogens. The effect of antibiotic treatment (daily for 3 days with subcutaneous saline [control], subcutaneous clindamycin, oral tebipenem pivoxil alone and in combination with CS319-piv-Sac, or oral CS319-piv-Sac) on the intestinal microbiota was assessed by culture for enterococci and facultative Gram-negative bacilli and by 16S rRNA amplicon sequencing. Mice were also challenged with 10,000 colony-forming units (CFU) of multidrug-resistant (MDR) strain Klebsiella pneumoniae blaNDM-1, 6 h after the second dose. The concentrations of the MDR K. pneumoniae in stool were measured on days 1, 3, and 6 after challenge. In comparison to saline controls, clindamycin (P = 0.001) and tebipenem pivoxil plus CS319-piv-SAc (P = 0.02) treatment resulted in significant changes in the alpha diversity patterns, whereas tebipenem pivoxil and CS319-piv-SAc individual treatments did not (P > 0.05). Moreover, clindamycin treatment resulted in substantial overgrowth of MDR K. pneumoniae (mean concentration after 6 days of infection, 6.1 vs 2.9 log10 CFU/g stool), whereas the other treatments did not (≤3.6 log10 CFU/g). Although tebipenem pivoxil alone or in combination with an MBL inhibitor, CS319, caused alteration of the mice intestinal microbiota, neither treatment promoted overgrowth of carbapenem-resistant K. pneumoniae.IMPORTANCEIn this work, we used a mouse model to determine the impact of tebipenem pivoxil alone and in combination with a prodrug of an experimental metallo-β-lactamase inhibitor, CS319, on the intestinal microbiota and on the establishment of colonization with carbapenem-resistant Klebsiella pneumoniae. We found that while treatment with tebipenem pivoxil plus the prodrug of CS319 caused alteration of the intestinal microbiota, it did not promote the overgrowth of carbapenem-resistant K. pneumoniae. Although additional studies are needed to examine the impact of tebipenem pivoxil treatment on other multidrug-resistant Gram-negative bacilli, Clostridioides difficile, and Candida spp., our study is a step forward in the understanding of the potential effect of this oral carbapenem on the indigenous microbiota of the colon and on the promotion of colonization by pathogens.
Keywords: carbapenem-resistant Enterobacterales; gut microbiome; intestinal colonization; metallo-β-lactamase inhibitor; tebipenem.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Effect of tebipenem pivoxil hydrobromide on the normal gut microbiota of a healthy adult population in Sweden: a randomised controlled trial.Lancet Microbe. 2024 Apr;5(4):e355-e365. doi: 10.1016/S2666-5247(23)00360-9. Epub 2024 Feb 29. Lancet Microbe. 2024. PMID: 38432233 Clinical Trial.
-
Antibacterial Properties of Tebipenem Pivoxil Tablet, a New Oral Carbapenem Preparation against a Variety of Pathogenic Bacteria in Vitro and in Vivo.Molecules. 2016 Jan 6;21(1):62. doi: 10.3390/molecules21010062. Molecules. 2016. PMID: 26751436 Free PMC article.
-
Effect of Ceftaroline, Ceftazidime/Avibactam, Ceftolozane/Tazobactam, and Meropenem/Vaborbactam on Establishment of Colonization by Vancomycin-Resistant Enterococci and Klebsiella pneumoniae in Mice.Pathog Immun. 2024 Sep 24;9(2):194-204. doi: 10.20411/pai.v9i2.711. eCollection 2024. Pathog Immun. 2024. PMID: 39345792 Free PMC article.
-
Tebipenem pivoxil hydrobromide-No PICC, no problem!Pharmacotherapy. 2021 Sep;41(9):748-761. doi: 10.1002/phar.2614. Epub 2021 Aug 17. Pharmacotherapy. 2021. PMID: 34370326 Review.
-
Microbiology of Meropenem-Vaborbactam: A Novel Carbapenem Beta-Lactamase Inhibitor Combination for Carbapenem-Resistant Enterobacterales Infections.Infect Dis Ther. 2020 Dec;9(4):757-767. doi: 10.1007/s40121-020-00350-1. Epub 2020 Oct 5. Infect Dis Ther. 2020. PMID: 33017041 Free PMC article. Review.
References
-
- Abujamel T, Cadnum JL, Jury LA, Sunkesula VCK, Kundrapu S, Jump RL, Stintzi AC, Donskey CJ. 2013. Defining the vulnerable period for re-establishment of Clostridium difficile colonization after treatment of C. difficile infection with oral vancomycin or metronidazole. PLoS One 8:e76269. doi:10.1371/journal.pone.0076269 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

