Mizoribine or Cyclophosphamide for Lupus Nephritis: A Randomized Clinical Trial
- PMID: 40085084
- PMCID: PMC11909609
- DOI: 10.1001/jamanetworkopen.2025.0648
Mizoribine or Cyclophosphamide for Lupus Nephritis: A Randomized Clinical Trial
Abstract
Importance: Lupus nephritis is typically treated with intravenous cyclophosphamide, which is associated with serious adverse effects. Oral mizoribine may be an alternative for induction therapy of lupus nephritis. However, large-scale, long-term, randomized clinical studies of mizoribine are lacking.
Objective: To assess the efficacy and safety of oral mizoribine vs intravenous cyclophosphamide as induction therapy for Chinese patients with lupus nephritis.
Design, setting, and participants: This prospective, multicenter, parallel-group, open-label, phase 3 randomized clinical trial recruited patients with class III, III+V, IV, IV+V, or V lupus nephritis aged 18 to 70 years from 40 centers in China. Inclusion criteria included 24-hour urinary protein level of 1.0 g or higher and systemic lupus erythematosus disease activity index of 8 or higher. The first patient was enrolled on November 29, 2014, and the study finished March 14, 2019. The follow-up period was 52 weeks. Data were analyzed from September 4, 2019, to January 21, 2020.
Interventions: Oral mizoribine (50 mg, 3 times a day) or cyclophosphamide (6 intravenous doses at 0.5-1.0 g/m2 body surface area, with a maximum dose of 1.0 g/d) for 52 weeks plus oral glucocorticoid.
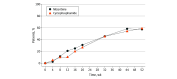
Main outcomes and measures: Total remission rate (complete remission rate plus partial remission rate) after 52 weeks (prespecified).
Results: A total of 250 patients were randomized, and 243 patients (mean [SD] age, 34.6 [10.7] years, 213 women [87.7%]) were treated (123 patients [50.6%] in the mizoribine group and 120 patients [49.4%] in the cyclophosphamide group). The total remission rate at 52 weeks was 66.1% (76 of 115 patients) in the mizoribine group and 76.8% (86 of 112 patients) in the cyclophosphamide group, and the relative risk ratio (mizoribine vs cyclophosphamide) was 0.861 (95% CI, 0.729-1.016). The lower limit of this 2-sided 95% CI was greater than the noninferiority margin of 0.726, indicating that mizoribine was noninferior to cyclophosphamide. Changes in other immune parameters and kidney function were generally similar between the groups. The incidence of any treatment-related treatment-emergent adverse events was 80.5% (99 of 123 patients) in the mizoribine group and 78.7% (96 of 122 patients) in the cyclophosphamide group, and the most frequent adverse event in both groups was upper respiratory tract infection (41 patients [33.3%] and 37 patients [30.3%], respectively).
Conclusions and relevance: This randomized clinical trial shows that compared with intravenous cyclophosphamide, oral mizoribine was noninferior and well tolerated when used with glucocorticoid for induction therapy of active lupus nephritis. Mizoribine can be used as an alternative to intravenous cyclophosphamide as induction therapy for lupus nephritis.
Trial registration: ClinicalTrials.gov Identifier: NCT02256150.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

