Triggering and modulation of a complex behavior by a single peptidergic command neuron in Drosophila
- PMID: 40085652
- PMCID: PMC11929487
- DOI: 10.1073/pnas.2420452122
Triggering and modulation of a complex behavior by a single peptidergic command neuron in Drosophila
Abstract
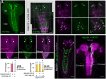
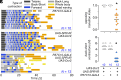
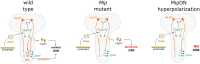
At the end of their growth phase, Drosophila larvae remodel their bodies, glue themselves to a substrate, and harden their cuticle in preparation for metamorphosis. This process-termed pupariation-is triggered by a surge in the hormone ecdysone. Substrate attachment is achieved by a pupariation subprogram called glue expulsion and spreading behavior (GSB). An epidermis-to-CNS Dilp8-Lgr3 relaxin signaling event that occurs downstream of ecdysone is critical for unlocking progression of the pupariation motor program toward GSB, but the factors and circuits acting downstream of Lgr3 signaling remain unknown. Here, using cell-type-specific RNA interference and behavioral monitoring, we identify Myoinhibiting peptide (Mip) as a neuromodulator of multiple GSB action components, such as tetanic contraction, peristaltic contraction alternation, and head-waving. Mip is required in a pair of brain descending neurons, which act temporally downstream of Dilp8-Lgr3 signaling. Mip modulates GSB via ventral nerve cord neurons expressing its conserved receptor, sex peptide receptor (SPR). Silencing of Mip descending neurons by hyperpolarization completely abrogates GSB, while their optogenetic activation at a restricted competence time window triggers GSB-like behavior. Hence, Mip descending neurons have at least two functions: to act as GSB command neurons and to secrete Mip to modulate GSB action components. Our results provide insight into conserved aspects of Mip-SPR signaling in animals, reveal the complexity of GSB control, and contribute to the understanding of how multistep innate behaviors are coordinated in time and with other developmental processes through command neurons and neuropeptidergic signaling.
Keywords: Drosophila; innate behavior; neuromodulation; neuropeptide; relaxin.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures





References
-
- Marder E., Bucher D., Schulz D. J., Taylor A. L., Invertebrate central pattern generation moves along. Curr. Biol. 15, R685–R699 (2005). - PubMed
MeSH terms
Substances
Grants and funding
- PTDC/BIA-BID/31071/2017 EXPL/BIA-BID/1524/2021 EXPL/BIA-COM/1296/2021 PTDC/MED-NEU/30753/2017/MEC | Fundação para a Ciência e a Tecnologia (FCT)
- PICT 2017-0254 PICT 2020-01568/Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación (agenciaidiar)
- P40 OD018537/OD/NIH HHS/United States
- PIP11220150100182CO/Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET)
- DFG WE 2652/4-1 DFG WE 2652/4-2/Deutsche Forschungsgemeinschaft (DFG)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

