QRICH1 mediates an intracellular checkpoint for CD8+ T cell activation via the CARD11 signalosome
- PMID: 40085689
- PMCID: PMC12291065
- DOI: 10.1126/sciimmunol.adn8715
QRICH1 mediates an intracellular checkpoint for CD8+ T cell activation via the CARD11 signalosome
Abstract
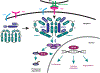
Antigen receptor signaling pathways that control lymphocyte activation depend on signaling hubs and negative regulatory proteins to fine-tune signaling outputs to ensure host defense and avoid pathogenic responses. Caspase recruitment domain-containing protein 11 (CARD11) is a critical signaling scaffold that translates T cell receptor (TCR) triggering into the activation of nuclear factor κB (NF-κB), c-Jun N-terminal kinase (JNK), mechanistic target of rapamycin (mTOR), and Akt. Here, we identify glutamine-rich protein 1 (QRICH1) as a regulator of CARD11 signaling that mediates an intracellular checkpoint for CD8+ T cell activation. QRICH1 associates with CARD11 after TCR engagement and negatively regulates CARD11 signaling to NF-κB. QRICH1 binding to CARD11 is controlled by an autoregulatory intramolecular interaction between QRICH1 domains of previously uncharacterized function. QRICH1 controls the antigen-induced activation, proliferation, and effector status of CD8+ T cells by regulating numerous genes critical for CD8+ T cell function. Our results define a component of antigen receptor signaling circuitry that fine-tunes effector output in response to antigen recognition.
Conflict of interest statement
Figures







References
-
- Gaud G, Lesourne R, Love PE, Regulatory mechanisms in T cell receptor signalling. Nat. Rev. Immunol. 18, 485–497 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

