Senolytic treatment for low back pain
- PMID: 40085710
- PMCID: PMC11908501
- DOI: 10.1126/sciadv.adr1719
Senolytic treatment for low back pain
Abstract
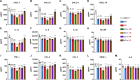
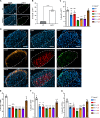
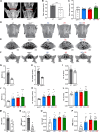
Senescent cells (SnCs) accumulate because of aging and external cellular stress throughout the body. They adopt a senescence-associated secretory phenotype (SASP) and release inflammatory and degenerative factors that actively contribute to age-related diseases, such as low back pain (LBP). The senolytics, o-vanillin and RG-7112, remove SnCs in human intervertebral discs (IVDs) and reduce SASP release, but it is unknown whether they can treat LBP. sparc-/- mice, with LBP, were treated orally with o-vanillin and RG-7112 as single or combination treatments. Treatment reduced LBP and SASP factor release and removed SnCs from the IVD and spinal cord. Treatment also lowered degeneration scores in the IVDs, improved vertebral bone quality, and reduced the expression of pain markers in the spinal cord. Together, our data suggest RG-7112 and o-vanillin as potential disease-modifying drugs for LBP and other painful disorders linked to cell senescence.
Figures



References
-
- Jeon O. H., Kim C., Laberge R. M., Demaria M., Rathod S., Vasserot A. P., Chung J. W., Kim D. H., Poon Y., David N., Baker D. J., van Deursen J. M., Campisi J., Elisseeff J. H., Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med. 23, 775–781 (2017). - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

