Endothelial cell activation enhances thromboinflammation in vaccine-induced immune thrombotic thrombocytopenia
- PMID: 40085945
- PMCID: PMC12181003
- DOI: 10.1182/bloodadvances.2024014165
Endothelial cell activation enhances thromboinflammation in vaccine-induced immune thrombotic thrombocytopenia
Abstract
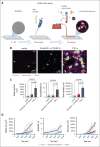
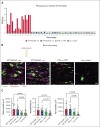
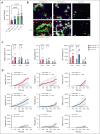
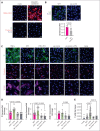
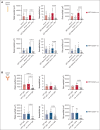
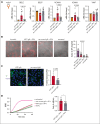
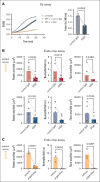
Vaccine-induced immune thrombotic thrombocytopenia (VITT) is a rare but serious complication of the ChAdOx1 nCOV-19 vaccine. In Australia, the diagnosis of VITT required the detection of antibodies against platelet factor 4 (PF4) in plasma using a PF4/polyanion enzyme-linked immunosorbent assay (ELISA). Half of the patients who fulfilled the clinical criteria for VITT tested positive when using this ELISA and another third tested positive when using platelet activation assays, highlighting limitations in the assays used for VITT. Using a microfluidic device coated with endothelial cells, the Endo-chip, we measured the effects of serum and immunoglobulin G (IgG) from patients with clinical VITT on endothelial thromboinflammation. Our cohort comprised 40 patients (21 ELISA-positive and 19 ELISA-negative patients as measured by PF4/polyanion ELISA), 12 vaccinated patients with venous thromboembolism without VITT, and 17 individuals who received the ChAdOx1 vaccine without adverse events (vax controls). Treatment with VITT serum, plasma, or IgG increased endothelial tissue factor (TF) expression and activity. Perfusion of blood from healthy donors labelled with fluorescent antibodies against platelets, neutrophils, and fibrin through Endo-chips treated with VITT serum or IgG induced a twofold to threefold increase in platelet, neutrophil, and fibrin deposition. Thromboinflammation was enhanced with addition of PF4 and reduced with an inhibitory antibody against TF. We conclude that endothelial activation contributes to thromboinflammation in patients with clinical features of VITT. The Endo-chip offers a platform for the study of endothelial responses in immune thrombosis.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

References
-
- Warkentin TE, Baskin-Miller J, Raybould AL, et al. Adenovirus-associated thrombocytopenia, thrombosis, and VITT-like antibodies. N Engl J Med. 2023;389(6):574–577. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

