Subgroup analysis of older patients ≥60 years with diffuse large B-cell lymphoma in the phase 3 POLARIX study
- PMID: 40085955
- PMCID: PMC12143816
- DOI: 10.1182/bloodadvances.2024014707
Subgroup analysis of older patients ≥60 years with diffuse large B-cell lymphoma in the phase 3 POLARIX study
Abstract
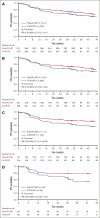
In the phase 3 POLARIX study, Pola-R-CHP (polatuzumab vedotin plus rituximab, cyclophosphamide, doxorubicin, and prednisone) improved progression-free survival (PFS) vs R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) in patients with previously untreated diffuse large B-cell lymphoma (DLBCL). This post hoc subgroup analysis of POLARIX evaluated the efficacy and safety of Pola-R-CHP vs R-CHOP in older patients aged ≥60, ≥65, ≥70, and ≥75 years. As of 15 June 2022 (median follow-up, 40 months), 629 patients aged ≥60 years were included (Pola-R-CHP, n = 311; R-CHOP, n = 318). Clinically meaningful improvements in PFS with Pola-R-CHP vs R-CHOP were observed across all age groups, particularly in patients aged ≥70 years whereby the risk of disease progression, relapse, or death was reduced by 37% (unstratified hazard ratio [HR], 0.63; 95% confidence interval [CI], 0.41-0.96). In patients aged ≥60 years, overall survival was similar with Pola-R-CHP vs R-CHOP (unstratified HR, 0. 99; 95% CI, 0.67-1.47). Safety profiles were similar for Pola-R-CHP vs R-CHOP among patients aged ≥60 years, including rates of grade 3 to 4 adverse events (AEs; 62.7% vs 61.5%), grade 3 to 5 infections (15.0% vs 12.9%), and grade 5 AEs (3.6% vs 3.2%); no novel toxicities were reported. Incidence of grade 3 to 4 febrile neutropenia was higher with Pola-R-CHP than R-CHOP (16.3% vs 7.6%), highlighting the importance of granulocyte colony-stimulating factor prophylaxis in older patients receiving Pola-R-CHP. The benefit-risk profile favored Pola-R-CHP vs R-CHOP in older patients with previously untreated DLBCL. This trial was registered at www.ClinicalTrials.gov as #NCT03274492.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: B.H. has served in a consultancy or advisory role for ADC Therapeutics, BeiGene, and Janssen; has acted in a leadership role for F. Hoffmann-La Roche Ltd/Genentech Inc; and has received research funding from Juno/Bristol Myers Squibb (BMS), and F. Hoffmann-La Roche Ltd/Genentech, Inc. P.M.R. has served in a consultancy or advisory role for Kite Pharma (a Gilead company) and Caribou Biosciences; has received honoraria from Kite Pharma; and has received research funding from Seagen, Genentech Inc, and F. Hoffmann-La Roche Ltd. L.H.S. has received grants or contracts from Cargo Therapeutics; has received honoraria from F. Hoffmann-La Roche Ltd/Genentech Inc, AbbVie, Janssen, Incyte, BeiGene, AstraZeneca, BMS/Celgene, Genmab, Merck, Seagen, and Kite/Gilead; and has received research funding from F. Hoffmann-La Roche Ltd/Genentech, Inc. J.P.S. has served in a consulting or advisory role for AbbVie, AstraZeneca, BeiGene, BMS, Lilly, Merck, and Genentech Inc. M.H. has served in a consulting or advisory role for F. Hoffmann-La Roche Ltd, Takeda, Otsuka, Gilead Sciences, Menarini, and BeiGene; and has received travel, accommodations, or expenses from Takeda. D.M. has received honoraria from Merck Sharp and Dohme (MSD), Zenyaku, BMS Japan, AstraZeneca, Nippon Shinyaku, Takeda, Janssen, Eisai, Celgene, Kyowa Kirin, Genma, Novartis, Ono Pharmaceutical, Sanofi, Mundipharma, AbbVie, and Chugai Pharma; and has received research funding from Astellas Pharma, AstraZeneca, MSD, Otsuka, Novartis, Kyowa Kirin, AbbVie, Sanofi, Symbio, BMS, Chugai Pharma, Ono Pharmaceutical, Janssen, Pfizer, Genmab, Zenkayu, Eisai, Takeda, and Taiho. F.S. has received payment for presentation at F. Hoffmann-La Roche Ltd/CH; has received travel, accommodation, or expenses from F. Hoffmann-La Roche Ltd (International Conference on Malignant Lymphoma [ICML] conference 2022); and has received payment for participation on an advisory board by F. Hoffmann-La Roche Ltd/CH. C.L.B. has served as an employee of Genentech, Inc/F. Hoffmann-La Roche Ltd; reports stock and other ownership interests from F. Hoffmann-La Roche Ltd; has received research funding from Epizyme, Autolus, Roche, and Vincerx; has received consulting fees from BMS, Seagen, Kite, Karyopharma, TG Therapeutics, ADC Therapeutics, AbbVie, Genentech, Treeline Biosciences; and has received honoraria from Dava Oncology, Touch Independent Medical Education (TouchIME), and Medscape. J.H. reports stock and other ownership interests from F. Hoffmann-La Roche Ltd. D.S. has served as an employee of F. Hoffmann-La Roche Ltd; and reports stock and other ownership from F. Hoffmann-La Roche Ltd. C.L. has served as an employee of Genentech, Inc/F. Hoffmann-La Roche Ltd, and reports stock and other ownership from F. Hoffmann-La Roche Ltd. M.S. reports stock and other ownership interests from Genentech, Inc/F. Hoffmann-La Roche Ltd. H.T. has received support for the present manuscript from F. Hoffmann-La Roche Ltd; has received honoraria from F. Hoffmann-La Roche Ltd and Incyte; has received travel, accommodation, or expenses from F. Hoffmann-La Roche Ltd and Janssen; has received honoraria from BMS and F. Hoffmann-La Roche Ltd; and has received payment for participation on an advisory board by BMS and Karyopharm. The remaining authors declare no competing financial interests.
The current affiliation for J.H. is BeOne Medicine, San Carlos, CA.
The current affiliation for C.L. is Gilead Sciences, Foster City, CA.
Figures


References
-
- National Institute of Health and Care Excellence Tisagenlecleucel for treating relapsed or refractory diffuse large B-cell lymphoma after 2 or more systemic therapies. https://www.nice.org.uk/guidance/ta933 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

