Factors associated with survival after allogeneic transplantation for myeloid neoplasms harboring TP53 mutations
- PMID: 40085959
- PMCID: PMC12274816
- DOI: 10.1182/bloodadvances.2024015335
Factors associated with survival after allogeneic transplantation for myeloid neoplasms harboring TP53 mutations
Abstract
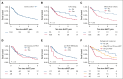
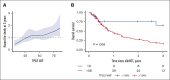
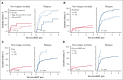
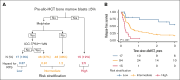
Allogeneic hematopoietic stem cell transplant (alloHCT) is considered for all patients with myeloid neoplasms (MNs) harboring TP53 mutations (TP53mut). The aim of this international study across 7 transplant centers in the United States and Australia was to identify factors associated with improved post-alloHCT survival. Of 134 TP53mut MN cases who underwent alloHCT, 80% harbored complex karyotype; 94% of TP53 variants were localized to the DNA-binding domain (DBD). Most common comutations were ASXL1 (7%), TET2 (7%), and DNMT3A (6%). Median post-HCT survival was 1.03 years, and overall survival (OS) at 1 year, 2 years, and 3 years was 51.4%, 35.1%, and 25.1%, respectively. A total of 103 cases (76.9%) met the International Consensus Classification (ICC) criteria for MN with mutated TP53 (referred to as ICC-defined TP53mut MN hereafter). The 3-year OS of ICC-defined TP53mut was significantly shorter compared with that of other TP53mut MNs (3-year OS, 16.9% vs 54.9%; P = .002). ICC-defined TP53mut MNs was independently associated with inferior OS (hazard ratio [HR], 2.62; P = .019). The presence of non-DBD TP53mut only (HR, 3.40; P = .005), DNMT3A comutation (HR, 2.64; P = .016), and pre-alloHCT bone marrow blasts ≥5% (HR, 2.76; P = .006) was independently associated with inferior relapse-free survival (RFS), whereas melphalan-based conditioning was associated with superior RFS (HR, 0.52; P = .005). Combining these variables, we constructed a hierarchical model that stratified patients into low-, intermediate-, and high-risk categories with 1-year RFS of 81.3%, 31.3%, and 6.7%, respectively (P < .001). In conclusion, a subset of MN harboring TP53mut who have low blasts pre-alloHCT and received melphalan-based conditioning derived long-term benefit from alloHCT.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: M.V.S. reports receiving research funding to institution from Astellas, AbbVie, Celgene, and Marker Therapeutics. A.A.P. reports receiving research funding from Pfizer, Sumitomo, and Kronos Bio, and honoraria from AbbVie, Bristol Myers Squibb, and Sobi. M.A.K.-D. reports receiving research/grant from Bristol Myers Squibb, Novartis, and Pharmacyclics, and lecture/honorarium from Kite Pharma. The remaining authors declare no competing financial interests.
Figures


References
-
- Lane DP. Cancer. p53, guardian of the genome. Nature. 1992;358(6381):15–16. - PubMed
-
- Versluis J, Saber W, Tsai HK, et al. Allogeneic hematopoietic cell transplantation improves outcome in myelodysplastic syndrome across high-risk genetic subgroups: genetic analysis of the Blood and Marrow Transplant Clinical Trials Network 1102 Study. J Clin Oncol. 2023;41(28):4497–4510. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

