Mature megakaryocytes acquire immune characteristics in a mouse model of aplastic anemia
- PMID: 40086077
- PMCID: PMC12268570
- DOI: 10.1182/bloodadvances.2024015621
Mature megakaryocytes acquire immune characteristics in a mouse model of aplastic anemia
Abstract
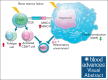
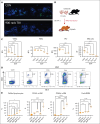
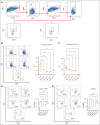
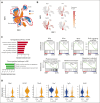
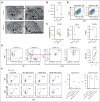
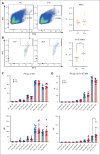
Megakaryocytes (MKs) serve diverse roles beyond platelet production, including hematopoietic stem cell maintenance and immune response modulation. In our mouse model of immune bone marrow failure (BMF), we observed the unexpected persistence of MKs despite thrombocytopenia. These MKs exhibited heightened expression of immune activation markers, such as IA-IE and CD53, compared with MKs from healthy controls. Single-cell RNA sequencing analysis (scRNA-seq) revealed upregulation of immune response pathways and downregulation of pathways related to platelet function and homeostasis in MKs from animals with marrow failure (BMF). Electron microscopy demonstrated that these MKs had fewer cytoplasmic extensions, reduced α-granules, and a less developed demarcation membrane system. MKs from BMF animals had reduced ability to produce platelets compared with normal control MKs. Interestingly, when cocultured with BMF-derived T cells, MKs from healthy mice acquired immune characteristics. Functionally, MKs from BMF mice suppressed hematopoietic stem cell colony formation in coculture experiments. Mechanistically, these MKs appeared to act as antigen-presenting cells, capable of T-cell activation. Notably, similar immune activation of MKs was observed in patients with aplastic anemia through scRNA-seq. These findings highlight the immune functions of mature MKs in an alloimmune model of BMF, with potential implications for human aplastic anemia and related hematologic disorders.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures





References
-
- Kordasti S, Marsh J, Al-Khan S, et al. Functional characterization of CD4+ T cells in aplastic anemia. Blood. 2012;119(9):2033–2043. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

