Telomere length in offspring is determined by mitochondrial-nuclear communication at fertilization
- PMID: 40087268
- PMCID: PMC11909127
- DOI: 10.1038/s41467-025-57794-7
Telomere length in offspring is determined by mitochondrial-nuclear communication at fertilization
Abstract
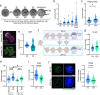
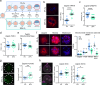
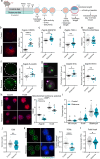
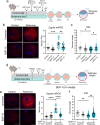
The initial setting of telomere length during early life in each individual has a major influence on lifetime risk of aging-associated diseases; however there is limited knowledge of biological signals that regulate inheritance of telomere length, and whether it is modifiable is not known. We now show that when mitochondrial activity is disrupted in mouse zygotes, via exposure to 20% O2 or rotenone, telomere elongation between the 8-cell and blastocyst stage is impaired, with shorter telomeres apparent in the pluripotent Inner Cell Mass (ICM) and persisting after organogenesis. Identical defects of elevated mtROS in zygotes followed by impaired telomere elongation, occurred with maternal obesity or advanced age. We further demonstrate that telomere elongation during ICM formation is controlled by mitochondrial-nuclear communication at fertilization. Using mitochondrially-targeted therapeutics (BGP-15, MitoQ, SS-31, metformin) we demonstrate that it is possible to modulate the preimplantation telomere resetting process and restore deficiencies in neonatal telomere length.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Y.E.W. and R.L.R. are inventors on a patent application (PCT/US23/69908) related to this work. R.L.R. is a consultant with Mitochon Technologies and Vitaleon Pharma. M.A.F. is a shareholder and consultant with N-Gene Research Laboratories Inc and is founder and shareholder of Celesta Therapeutics. H.H.S. is the inventor of SS-31 (USPTO 7,576,601; August 18, 2009). The remaining authors declare no competing interests.
Figures

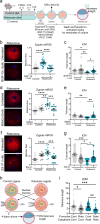
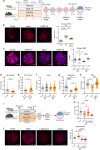
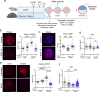
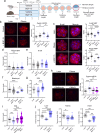
References
-
- Blackburn, E. H., Epel, E. S. & Lin, J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science (N. Y., NY)350, 1193–1198 (2015). - PubMed
-
- D’Mello, M. J. et al. Association between shortened leukocyte telomere length and cardiometabolic outcomes: systematic review and meta-analysis. Circ. Cardiovasc Genet.668, 82–90 (2015). - PubMed
MeSH terms
Substances
Grants and funding
- APP1130364/Department of Health | National Health and Medical Research Council (NHMRC)
- APP1117975/Department of Health | National Health and Medical Research Council (NHMRC)
- APP1194141/Department of Health | National Health and Medical Research Council (NHMRC)
- 20669397/Channel 7 Children's Research Foundation (CRF)
LinkOut - more resources
Full Text Sources
Miscellaneous

