A highly stable lyophilized mRNA vaccine for Herpes Zoster provides potent cellular and humoral responses
- PMID: 40087280
- PMCID: PMC11909110
- DOI: 10.1038/s41541-025-01093-1
A highly stable lyophilized mRNA vaccine for Herpes Zoster provides potent cellular and humoral responses
Abstract
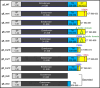
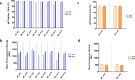
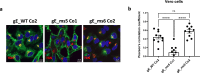
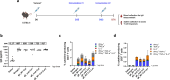
Herpes zoster (HZ) is a painful vesicular rash that occurs upon varicella-zoster virus (VZV) reactivation in older adults and immunocompromised individuals. Although there is currently an approved vaccine for the prevention of shingles, its administration is commonly associated with high reactogenicity. This highlights the need to develop new vaccine alternatives with long lasting immunity and improved tolerability upon administration. In the present study, 10 different vaccine candidate designs using two different codon optimizations targeting the VZV glycoprotein E (gE) were generated. A subset of mRNA constructs were formulated into lipid nanoparticles and assessed for their ability to induce specific cellular and humoral immune responses following vaccination in mice. Notably, the selected mRNA vaccine candidates induced high levels of antibodies and robust CD4+ but also CD8+ immune responses. Moreover, we showed that our alternate lyophilized vaccine provides comparable immunogenicity to current liquid frozen formulations and is stable under long-term storage conditions.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The study was funded by Pfizer Inc. Pfizer was involved in the design, analysis, and interpretation of the data in this research study, the writing of this report, and the decision to publish. The results in this manuscript are part of a collaboration between BioNTech and Pfizer. All authors are current or former employees of Pfizer Inc., and may, consequently, be shareholders. F.D., R.M.M., A.S. and P.S.A. are listed as inventors on a patent application related to nucleoside-modified RNA LNP vaccines encoding varicella zoster virus.
Figures


References
-
- Harpaz, R. & Leung, J. W. The Epidemiology of Herpes Zoster in the United States During the Era of Varicella and Herpes Zoster Vaccines: Changing Patterns Among Older Adults. Clin. Infect. Dis.69, 341–344 (2019). - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

