SIRT3-PINK1-PKM2 axis prevents osteoarthritis via mitochondrial renewal and metabolic switch
- PMID: 40087281
- PMCID: PMC11909255
- DOI: 10.1038/s41413-025-00413-4
SIRT3-PINK1-PKM2 axis prevents osteoarthritis via mitochondrial renewal and metabolic switch
Abstract
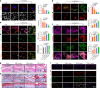
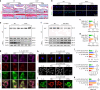
Maintaining mitochondrial homeostasis is critical for preserving chondrocyte physiological conditions and increasing resistance against osteoarthritis (OA). However, the underlying mechanisms governing mitochondrial self-renewal and energy production remain elusive. In this study, we demonstrated mitochondrial damage and aberrant mitophagy in OA chondrocytes. Genetically overexpressing PTEN-induced putative kinase 1 (PINK1) protects against cartilage degeneration by removing defective mitochondria. PINK1 knockout aggravated cartilage damage due to impaired mitophagy. SIRT3 directly deacetylated PINK1 to promote mitophagy and cartilage anabolism. Specifically, PINK1 phosphorylated PKM2 at the Ser127 site, preserving its active tetrameric form. This inhibited nuclear translocation and the interaction with β-catenin, resulting in a metabolic shift and increased energy production. Finally, a double-knockout mouse model demonstrated the role of the SIRT3-PINK1-PKM2 axis in safeguarding the structural integrity of articular joints and improving motor functions. Overall, this study provides a novel insight into the regulation of mitochondrial renewal and metabolic switches in OA.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures






References
-
- Zhang, Y. et al. Recharge of chondrocyte mitochondria by sustained release of melatonin protects cartilage matrix homeostasis in osteoarthritis. J. Pineal Res73, e12815 (2022). - PubMed
-
- Lemasters, J. J. Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res8, 3–5 (2005). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

