Clinical evaluation of a polarization-based optical noninvasive glucose sensing system
- PMID: 40087308
- PMCID: PMC11909277
- DOI: 10.1038/s41598-025-92515-6
Clinical evaluation of a polarization-based optical noninvasive glucose sensing system
Abstract
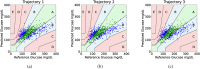
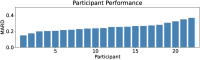
Diabetes affects millions in the US, causing elevated blood glucose levels that could lead to complications like kidney failure and heart disease. Recent development of continuous glucose monitors has enabled a minimally invasive option, but the discomfort and social factors highlight the need for noninvasive alternatives in diabetes management. We propose a portable noninvasive glucose sensing system based on the glucose's optical activity property which rotates linearly polarized light depending on its concentration level. To enable a portable form factor, a light trap mechanism is used to capture unwanted specular reflection from the palm and the enclosure itself. We fabricate four sensing prototypes and conduct a 363-day multi-session clinical evaluation in real-world settings. 30 participants are provided with a prototype for a 5-day home monitoring study, collecting on average 8 data points per day. We identify the error caused by differences between the sensing boxes and the participants' improper usage. We utilize a machine learning pipeline together with Bayesian Ridge Regressor models and multiple-step data processing techniques to deal with the noisy data. Over 95% of the predictions fall within Zone A (clinically accurate) or B (clinically acceptable) of the Consensus Error Grid with a 0.24 mean absolute relative differences.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: Forlenza conducts research supported by Medtronic, Dexcom, Abbott, Insulet, Tandem, Beta Bionics, and Lilly and has been a speaker/consultant/ad board member for Medtronic, Dexcom, Abbott, Insulet, Tandem, Beta Bionics, and Lilly. All other authors declare no competing interests. Ethical approval: All methods and protocols included in this study are approved by the Institutional Review Board of Barbara Davis Center for Diabetes at University of Colorado Anschutz Medical Campus (COMIRB #22-0074).
Figures




References
-
- Centers for Disease Control and Prevention. National Diabetes Statistics Report. https://www.cdc.gov/diabetes/php/data-research/index.html (2021). Accessed 30 July 2024.
-
- Group D. R. The diabetes control and complications trial (dcct): Design and methodologic considerations for the feasibility phase. Diabetes35, 530–545 (1986). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

