Lenvatinib plus pembrolizumab compared to carboplatin plus paclitaxel for carboplatin and paclitaxel pretreated, recurrent, or advanced endometrial cancer
- PMID: 40087652
- PMCID: PMC11909920
- DOI: 10.1186/s12916-025-03989-0
Lenvatinib plus pembrolizumab compared to carboplatin plus paclitaxel for carboplatin and paclitaxel pretreated, recurrent, or advanced endometrial cancer
Abstract
Background: Lenvatinib plus pembrolizumab has demonstrated improved survival compared with doxorubicin or paclitaxel monotherapy in patients with advanced or recurrent endometrial cancers (ECs). However, response rates to monotherapy are poor in recurrent settings. Herein, we performed a retrospective analysis using real-world data to compare the outcomes of lenvatinib plus pembrolizumab, carboplatin plus paclitaxel (PT), and doxorubicin for patients with PT-pretreated, advanced, or recurrent ECs.
Methods: We performed a multi-institutional retrospective analysis using de-identified electronic health record database (TriNetX) to compare lenvatinib plus pembrolizumab, carboplatin plus paclitaxel (PT), and doxorubicin outcomes in patients with PT-pretreated, advanced, or recurrent ECs. A 1:1 propensity score matching (PSM) was conducted. The primary outcome was the overall survival (OS) among treatment groups. The secondary outcome was the adverse event profile.
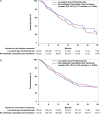
Results: Between January 2012 and September 2023, we identified 397 patients with PT-treated, advanced, or recurrent ECs who received lenvatinib plus pembrolizumab, and 469 patients receiving PT at a platinum-free interval of over 6 months. Following PSM, no significant difference in median OS was observed between the lenvatinib plus pembrolizumab and re-challenge PT groups (19.1 vs. 18.5 months, p = 0.60; hazard ratio: 1.08, 95% confidence interval 0.81-1.46). However, lenvatinib plus pembrolizumab provided better survival benefits than doxorubicin. Adverse event analysis showed more hypothyroidism, hypertension, and proteinuria with lenvatinib plus pembrolizumab, and more hematologic toxicities in both chemotherapy groups.
Conclusions: Lenvatinib plus pembrolizumab was not associated with improved survival when compared with re-challenge PT in patients with a platinum-free interval of over 6 months. Re-challenge PT remains a valid option for PT-treated, recurrent, or advanced ECs, especially in patients with a substantially long platinum-free interval.
Keywords: Carboplatin; Doxorubicin; Endometrial cancer; Lenvatinib; Paclitaxel; Pembrolizumab.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This work was conducted in compliance with the principles of the 1975 Declaration of Helsinki and was approved by the Ethics Committee of Taichung Veterans General Hospital (CE24073C). Consent to participate is waived in this study as TriNetX has recieved a waiver of informed consent from the Western Institutional Review Board. All data and statistical summaries were displayed in aggregated counts, and no identifiable information can be accessed. Consent for publication: All data were anonymized to protect individuals' confidentiality, and no personally identifiable information is accessible or shareable. Only aggregate data derived from the analyses will be published. As a result, informed consent from individual participants was waived for this study. Competing interests: The authors declare no competing interests.
Figures



References
-
- Price FV, Edwards RP, Kelley JL, Kunschner AJ, Hart LA. A trial of outpatient paclitaxel and carboplatin for advanced, recurrent, and histologic high-risk endometrial carcinoma: preliminary report. Semin Oncol. 1997;24(5 Suppl 15):S15-78-S15–82. - PubMed
-
- Miller DS, Filiaci VL, Mannel RS, Cohn DE, Matsumoto T, Tewari KS, et al. Carboplatin and Paclitaxel for Advanced Endometrial Cancer: Final Overall Survival and Adverse Event Analysis of a Phase III Trial (NRG Oncology/GOG0209). J Clin Oncol. 2020;38(33):3841–50. 10.1200/JCO.20.01076. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

