The Japanese Clinical Practice Guidelines for Management of Sepsis and Septic Shock 2024
- PMID: 40087807
- PMCID: PMC11907869
- DOI: 10.1186/s40560-025-00776-0
The Japanese Clinical Practice Guidelines for Management of Sepsis and Septic Shock 2024
Abstract
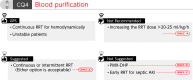
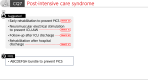
The 2024 revised edition of the Japanese Clinical Practice Guidelines for Management of Sepsis and Septic Shock (J-SSCG 2024) is published by the Japanese Society of Intensive Care Medicine and the Japanese Association for Acute Medicine. This is the fourth revision since the first edition was published in 2012. The purpose of the guidelines is to assist healthcare providers in making appropriate decisions in the treatment of sepsis and septic shock, leading to improved patient outcomes. We aimed to create guidelines that are easy to understand and use for physicians who recognize sepsis and provide initial management, specialized physicians who take over the treatment, and multidisciplinary healthcare providers, including nurses, physical therapists, clinical engineers, and pharmacists. The J-SSCG 2024 covers the following nine areas: diagnosis of sepsis and source control, antimicrobial therapy, initial resuscitation, blood purification, disseminated intravascular coagulation, adjunctive therapy, post-intensive care syndrome, patient and family care, and pediatrics. In these areas, we extracted 78 important clinical issues. The GRADE (Grading of Recommendations Assessment, Development and Evaluation) method was adopted for making recommendations, and the modified Delphi method was used to determine recommendations by voting from all committee members. As a result, 42 GRADE-based recommendations, 7 good practice statements, and 22 information-to-background questions were created as responses to clinical questions. We also described 12 future research questions.
Keywords: Evidence-based medicine; GRADE; Infection; Intensive care; Organ failure; Systematic review.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Competing interests: All committee members and working group members submitted disclosure forms of financial and academic conflict of interest (COI) prior to being requested to participate in individual activities. All COI were collected according to the guideline by the JSICM and the JAAM. Detailed information of COI and the roles in creating this clinical guideline are summarized in Additional file 4.
Figures







References
-
- Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. - PubMed
-
- Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–55. - PubMed
-
- Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Intensive Care Med. 2003;29:530–8. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

