Real-World Outcomes of Newly Diagnosed Multiple Myeloma Patients Treated Before the Era of Anti-CD38 Antibodies: The EMMY Cohort From 2017 to 2020
- PMID: 40087879
- PMCID: PMC11909396
- DOI: 10.1002/cam4.70619
Real-World Outcomes of Newly Diagnosed Multiple Myeloma Patients Treated Before the Era of Anti-CD38 Antibodies: The EMMY Cohort From 2017 to 2020
Abstract
Aims/background: Recent agents have profoundly reshaped the multiple myeloma (MM) landscape. Their real-world impacts need to be assessed over the long term.
Methods: EMMY is a non-interventional, prospective dynamic cohort, conducted in France, since 2017, with 900 patients enrolled each year. Newly diagnosed MM (NDMM) who initiated a treatment from 2017 to 2020 are here described.
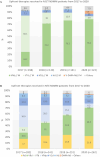
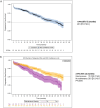
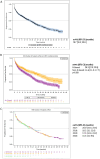
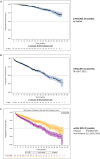
Results: A total of 1036 non-transplant eligible (NTE) patients (median age: 74.9 years) and 561 patients who received autologous stem cell transplantation (ASCT) (median age: 60.6 years) were enrolled. For ASCT patients, a shift in induction treatment from bortezomib-thalidomide-dexamethasone (VTd) (29.1%) to bortezomib-lenalidomide-dexamethasone (VRd) (55.1%) marked the period. Maintenance treatment with R after ASCT became a standard (75% of patients). In NTE patients, R-based regimens were increasingly used from 29.4% in 2017 (of whom Rd.: 17.0%, VRd: 10.6%) to 73.3% in 2020 (of whom Rd.: 21.8%, VRd: 48.5%). Median progression-free survival (mPFS) was 46.5 months (95% CI: 37.8-50.6) and 18.7 months (95% CI: 16.3-20.8) in ASCT and NTE patients, respectively. In the ASCT group, patients treated with and without R maintenance had a mPFS of 51.8 (95% CI: 44.1-NA) and 29.6 months (95% CI: 21.8-40.9), respectively. In the NTE group, the mPFS was 26.3 (95% CI: 21.9-30.9) and 14.6 months (95% CI: 11.9-17.7) in patients who received an R-based and non-R-based regimen, respectively. The estimated 48-month overall survival rates were 89% (95% CI: 84.5-92.2) and 63% (95% CI: 58.5-67.1) for ASCT and NTE patients, respectively.
Conclusions: The 2017-2020 period was marked by the expansion of R use in both NDMM ASCT and NTE patients.
Keywords: ASCT patients; NTE patients; first‐line therapy; multiple myeloma; real world.
© 2025 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
L.V. received honoraria from Janssen, BMS, Takeda, Sanofi, Pfizer; O.D. received honoraria from Janssen, BMS, GSK, Sanofi, Takeda, Roche and Gilead; A.P. received honoraria from Abbvie, Amgen, BMS, GSK, Janssen, Sanofi and Takeda; M.M. received honoraria from Janssen, Sanofi, GSK and Takeda; L.K. received honoraria from Amgen, BMS, Janssen, Takeda, Sanofi, Abbvie, Stemline and GSK; C.S. participate as expert in board of experts for BMS, Amgen, Janssen, Takeda and Sanofi; M.M. received honoraria from Janssen, Sanofi, BMS, Amgen, Takeda, GSK, Pfizer, Novartis, Jazz Pharmaceuticals, Stemline and Astellas; L.F. is a consultant for Pfizer, Roche, CSL Behring, Sobi, BioMarin Pharmaceutical, A.J. received honoraria from Abbvie, Pfizer and Janssen; S.M. is a consultant for AbbVie, Adaptative Biotechnology, Amgen, BMS, GSK, Janssen, Regeneron, Roche, Sanofi, Takeda; P.M. is a consultant and received honoraria from Janssen, BMS, Amgen, Abbvie, Sanofi and Takeda; L.C. et ZM are employees of IFM; C.H. received honoraria from Janssen, BMS, GSK, Takeda, Sanofi and Amgen; K.B.M. is a consultant and received honoraria for BMS, Janssen, Sanofi, Amgen and Abbvie. The other authors declare that they have no competing interests.
Figures

References
-
- Ferlay J., Colombet M., Soerjomataram I., et al., “Cancer Incidence and Mortality Patterns in Europe: Estimates for 40 Countries and 25 Major Cancers in 2018,” European Journal of Cancer 103 (2018): 356–387. - PubMed
-
- Moreau P., San Miguel J., Sonneveld P., et al., “Multiple Myeloma: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow‐Up,” Annals of Oncology 28, no. suppl_4 (2017): 52–61. - PubMed
-
- Siegel R. L., Miller K. D., and Jemal A., “Cancer Statistics, 2018,” CA: A Cancer Journal for Clinicians 68, no. 1 (2018): 7–30. - PubMed
-
- Dimopoulos M. A., Moreau P., Terpos E., et al., “EHA‐ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow‐Up†,” Annals of Oncology 32, no. 3 (2021): 309–322. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

