Hematologic and molecular responses to ropeginterferon alfa-2b therapy of polycythemia vera: 48-week results from a prospective study
- PMID: 40087986
- PMCID: PMC12141981
- DOI: 10.1002/ijc.35411
Hematologic and molecular responses to ropeginterferon alfa-2b therapy of polycythemia vera: 48-week results from a prospective study
Abstract
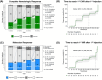
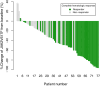
To prevent thrombosis in patients with polycythemia vera (PV), achieving a complete hematologic response (CHR) is highly recommended in practice. In addition, a reduced JAK2 V617F mutation burden is expected to have a disease-modifying effect, and its molecular response (MR) is currently of significant interest. This study aimed to assess the association between CHR and MR in patients with PV following treatment with ropeginterferon alfa-2b. This phase 2, single-arm, open-label, investigator-initiated trial was conducted at 16 sites in South Korea. Ninety-nine patients were treated with ropeginterferon alfa-2b subcutaneously every 2 weeks, at doses of 250 μg (week 1), 350 μg (week 3), and 500 μg (week 5), until week 48. CHRs were 27% (25/94), 46% (40/87), 56% (47/84), and 63% (51/81) at 12, 24, 36, and 48 weeks, respectively. The MR rates were 32% (28/88), 36% (29/81), 49% (38/77), and 57% (42/74) at 12, 24, 36, and 48 weeks, respectively. The Phi Coefficient for the association between CHR and MR was 0.6146 (p < .0001) at 48 weeks. In the subgroup analysis, patients with hydroxyurea resistance or intolerance, and those who were hydroxyurea-naïve, had similar results in terms of the CHR. In conclusion, CHR and MR were observed to be associated in patients with PV treated with ropeginterferon.
Keywords: CHR; MR; association; polycythemia vera; ropeginterferon alfa‐2b.
© 2025 The Author(s). International Journal of Cancer published by John Wiley & Sons Ltd on behalf of UICC.
Conflict of interest statement
SYL's institution has received research funding from PharmaEssentia. The other authors declare no conflicts of interest.
Figures
References
-
- Kralovics R, Passamonti F, Buser AS, et al. A gain‐of‐function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779‐1790. - PubMed
-
- Tefferi A, Barbui T. Polycythemia vera: 2024 update on diagnosis, risk‐stratification, and management. Am J Hematol. 2023;98:1465‐1487. - PubMed
-
- National Comprehensive Cancer Network . Myeloproliferative Neoplasms (Version 1.2024). 2024.
-
- Marchetti M, Vannucchi AM, Griesshammer M, et al. Appropriate management of polycythaemia vera with cytoreductive drug therapy: European LeukemiaNet 2021 recommendations. Lancet Haematol. 2022;9:e301‐e311. - PubMed
-
- Barbui T, Vannucchi AM, De Stefano V, et al. Ropeginterferon versus standard therapy for low‐risk patients with polycythemia vera. NEJM Evid. 2023;2:EVIDoa2200335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

