Effectiveness and safety of adjunctive cenobamate in people with focal-onset epilepsy: Interim results after 24-week observational period from the BLESS study
- PMID: 40088187
- PMCID: PMC12291020
- DOI: 10.1111/epi.18357
Effectiveness and safety of adjunctive cenobamate in people with focal-onset epilepsy: Interim results after 24-week observational period from the BLESS study
Abstract
Objective: Cenobamate is an antiseizure medication (ASM) with a dual mechanism of action that was recently approved for the treatment of focal seizures in adults. This analysis aimed to describe the outcomes at 12 and 24 weeks after starting cenobamate therapy in a real-world setting.
Methods: BLESS [NCT05859854] is an ongoing, observational, retrospective and prospective cohort study to evaluate the real-world effectiveness and safety of adjunctive cenobamate in adults with uncontrolled focal epilepsy. Subgroup analysis was performed in subjects with 2 to 3 previous ASMs (early users) and those with >3 previous ASMs (late users).
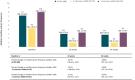
Results: The second interim analysis of the BLESS study included 388 participants with a median (interquartile range) age of 43.0 (31.0-54.0) years. They had a median of 6.0 (4.0-9.0) prior ASMs and a median of 7.2 (3.0-20.6) monthly seizures at baseline. The median monthly seizure frequency was reduced by 59.9% (19.2%-87.3%) from baseline to 24 weeks; 229 (59.0%) subjects had a ≥50% seizure frequency reduction, and 44 (11.3%) showed sustained seizure freedom. The proportion of participants taking ≤2 concomitant ASMs increased from 217 (56.5%) at baseline to 239 (65.7%) at 24 weeks. Among the early users (n = 76, 19.6%), the median reduction in monthly seizure frequency at 24 weeks was 78.0% (50.0-97.1%), and 76.3% of subjects had a ≥50% response rate. The frequency of adverse drug reactions (ADRs) was 5.3% and 23.4% in early and late users. The most frequent ADRs were somnolence, dizziness, and balance disorder; after the occurrence of ADRs, 63.5% of participants maintained the prescribed dose, and 5.2% permanently discontinued treatment.
Significance: Cenobamate was effective in reducing seizure frequency in a real-world setting and showed a manageable safety profile. The treatment with cenobamate also reduced the burden of concomitant ASMs in both early and late users.
Keywords: cenobamate; early use; epilepsy management; real‐world evidence; seizure reduction.
© 2025 IQVIA Solutions Italy SRL, Angelini Pharma SpA and The Author(s). Epilepsia published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
The authors declare the following financial interests/personal relationships, which may be considered as potential competing interests: Simona Lattanzi has received speaker or consultancy fees from Angelini Pharma, Eisai, GW Pharmaceuticals, Medscape, and UCB Pharma, and has served on advisory boards for Angelini Pharma, Arvelle Therapeutics, BIAL, Eisai, GW Pharmaceuticals, and Rapport Therapeutics. Fedele Dono has received speaker honoraria and travel grant from Eisai, Jazz Pharmaceuticals, and Angelini Pharma. Giuseppe d'Orsi has received speaker or consultancy fees from Angelini Pharma, Eisai, and UCB Pharma. Alfredo D'Aniello has received speaker honoraria from Angelini Pharma, UCB Pharma, and Eisai, and has participated in advisory board for Angelini Pharma. Mariangela Panebianco has received speaker or consultancy fees from Angelini Pharma, Eisai, and UCB Pharma. Paolo Bonanni has received speaker or consultancy fees from BIAL, Eisai, GW Pharmaceuticals, LivaNova, Lusofarmaco, Proveca, and Roche. Carlo Di Bonaventura has received consulting fees and honoraria from UCB Pharma, Eisai, GW Pharmaceuticals, BIAL, Angelini Pharma, Lusofarmaco, and Ecupharma. Antonio Gambardella has received speaker honoraria from Eisai, UCB Pharma, and Angelini Pharma. Federica Ranzato has received speaker fees from Angelini Pharma, Eisai, UCB Pharma, and LivaNova and has participated on an advisory board for Angelini Pharma. Giada Pauletto has received speaker's or consultancy fees from Eisai, Angelini Pharma, LivaNova, Lusofarmaco, and UCB Pharma. Elena Tartara has received speaker fees from Eisai and advisory board fees from Angelini Pharma. Angela La Neve has received speaker or consultancy fees from Eisai, Mylan, Sanofi, BIAL, GW Pharmaceuticals, UCB Pharma, Arvelle Therapeutics, Angelini Pharma, and Neuraxpharm. Francesca Bisulli has received consultancy fees from Angelini Pharma, UCB Pharma, Jazz Pharmaceuticals, and Eisai. Patrizia Pulitano, has received consulting fees or speaker honoraria from Angelini Pharma, UCB Pharma, and Eisai. Claudio Liguori has received research support and speaker honoraria from Angelini Pharma and has no other relevant conflict of interests related to this article. Vincenzo Belcastro has received consulting fees and honoraria from UCB Pharma, Angelini Pharma, Lusofarmaco, and Ecupharma. Chiara Pizzanelli has received consultancy fees from Angelini Pharma, Eisai, and UCB Pharma. Maurizio Elia has received speaker or consultancy fees from Angelini Pharma, Eisai, Lusofarmaco, Proveca, Takeda, and UCB Pharma. Rosaria Renna, has received speaker fees from Eisai. Daniela Audenino has received speaker or consultancy fees from Angelini Pharma and UCB Pharma. Loretta Giuliano has received speaker or consultancy fees from Eisai, Angelini Pharma, and Lusofarmaco. Rosita Galli has received speaker or consultancy fees from Angelini Pharma and Eisai. Gionata Strigaro has received fees from Eisai and Angelini Pharma. Monica Puligheddu has received speaker or consultancy fees from Angelini Pharma, Eisai, Lusofarmaco, Idorsia, Jazz Pharmaceuticals, Bioproject, Fidia, and UCB Pharma. Giovanni Boero has received speaker or consultancy fees from Eisai, Angelini Pharma, and UCB Pharma. Giovanni Falcicchio, has received speaker fees from Angelini Pharma. Michela Procaccini is an employee of Angelini Pharma, Italy. Valentina Villano is an employee of Angelini Pharma, Italy. Gabriele Camattari is an employee of Angelini Pharma, Italy. Fabiano Mele is an employee of IQVIA Solutions Italy SRL. Barbara Roncari is an employee of IQVIA Solutions Italy SRL. Giancarlo Di Gennaro has received speaker honoraria from Eisai, UCB Pharma, LivaNova, Lusofarmaco, and GW Pharmaceuticals; and has served on advisory boards for BIAL, Arvelle Therapeutics, Angelini Pharma, and UCB Pharma. The remaining authors have no conflicts of interest.
Figures


References
-
- World Health Organization . Epilepsy. 2024. https://www.who.int/en/news‐room/fact‐sheets/detail/epilepsy
-
- Sultana B, Panzini M‐A, Carpentier AV, Comtois J, Rioux B, Gore G, et al. Incidence and prevalence of drug‐resistant epilepsy: a systematic review and meta‐analysis. Neurology. 2021;96(17):805–817. - PubMed
-
- Asadi‐Pooya AA, Brigo F, Lattanzi S, Blumcke I. Adult epilepsy. Lancet. 2023;402(10399):412–424. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

