Necrosis-like cell death modes in heart failure: the influence of aetiology and the effects of RIP3 inhibition
- PMID: 40088261
- PMCID: PMC11976840
- DOI: 10.1007/s00395-025-01101-4
Necrosis-like cell death modes in heart failure: the influence of aetiology and the effects of RIP3 inhibition
Abstract
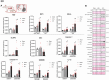
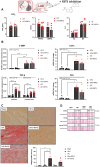
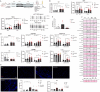
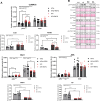
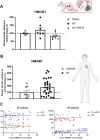
Since cell dying in heart failure (HF) may vary based on the aetiology, we examined the main forms of regulated necrosis, such as necroptosis and pyroptosis, in the hearts damaged due to myocardial infarction (MI) or pressure overload. We also investigated the effects of a drug inhibiting RIP3, a proposed convergent point for both these necrosis-like cell death modes. In rat hearts, left ventricular function, remodelling, pro-cell death, and pro-inflammatory events were investigated, and the pharmacodynamic action of RIP3 inhibitor (GSK'872) was assessed. Regardless of the HF aetiology, the heart cells were dying due to necroptosis, albeit the upstream signals may be different. Pyroptosis was observed only in post-MI HF. The dysregulated miRNAs in post-MI hearts were accompanied by higher levels of a predicted target, HMGB1, its receptors (TLRs), as well as the exacerbation of inflammation likely originating from macrophages. The RIP3 inhibitor suppressed necroptosis, unlike pyroptosis, normalised the dysregulated miRNAs and tended to decrease collagen content and affect macrophage infiltration without affecting cardiac function or structure. The drug also mitigated the local heart inflammation and normalised the higher circulating HMGB1 in rats with post-MI HF. Elevated serum levels of HMGB1 were also detected in HF patients and positively correlated with C-reactive protein, highlighting pro-inflammatory axis. In conclusion, in MI-, but not pressure overload-induced HF, both necroptosis and pyroptosis operate and might underlie HF pathogenesis. The RIP3-targeting pharmacological intervention might protect the heart by preventing pro-death and pro-inflammatory mechanisms, however, additional strategies targeting multiple pro-death pathways may exhibit greater cardioprotection.
Keywords: Heart failure; High mobility group box 1; Inflammation; Necroptosis; Pyroptosis; Receptor-interacting protein kinase 3.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare that they have no conflict of interest.
Figures





References
-
- Adameova A, Goncalvesova E, Szobi A, Dhalla NS (2016) Necroptotic cell death in failing heart: relevance and proposed mechanisms. Heart Fail Rev 21:213–221. 10.1007/s10741-016-9537-8 - PubMed
MeSH terms
Substances
Grants and funding
- APVV-20-0242/Agentúra na Podporu Výskumu a Vývoja
- APPV-15-0607/Agentúra na Podporu Výskumu a Vývoja
- NU21J-02-00039/Agentura Pro Zdravotnický Výzkum České Republiky
- NU22-01-00096/Agentura Pro Zdravotnický Výzkum České Republiky
- LX22NPO5104/National Institute for Research of Metabolic and Cardiovascular Diseases, Programme EXCELES
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

