Reactivity of aragonite with dicalcium phosphate facilitates removal of dental calculus
- PMID: 40088368
- PMCID: PMC11910395
- DOI: 10.1007/s10856-025-06867-6
Reactivity of aragonite with dicalcium phosphate facilitates removal of dental calculus
Abstract
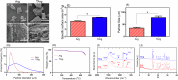
Dental calculus, a main contributor of periodontal diseases, is mostly composed of inorganic calcium phosphate species such as dicalcium phosphate, whitlockite, octa calcium phosphate, and hydroxyapatite. Under physiological pH 7.4, dicalcium phosphates can gradually interact with calcium carbonate to form hydroxyapatite. Therefore, we hypothesized that aragonite (Arg) could react with dental calculus, facilitating its removal. To assess the reactivity of Arg with dental calculus, we examined the changes in surface morphology, composition, and topography of Arg and dental calculus upon exposure to each other in an aqueous environment. The impact of Arg on the removal of dental calculus was assessed by brushing polished sections of dental calculus, enamel, and dentin with slurries of Arg and measuring the depth of abrasion using a stylus profilometer. Our results demonstrate that Arg can react with dental calculus in aqueous environment. This reaction increases calculus surface roughness which in turn facilitate dental calculus removal by brushing. Aragonite could be a promising abrasive for toothpaste design for management of dental calculus.
© 2025. The Author(s).
Conflict of interest statement
Compliance with ethical standards. Conflict of interest: FT declares that he holds share in the company Visionaturalab Inc., which holds IP related to the data presented in this manuscript. The authors declare no other competing interests. Ethics approval: This study was conformed to the ethical guidelines of the World Medical Association Declaration of Helsinki. The collection of dental and calculus specimens was approved by McGill University Health Centre Ethical Committee, Montreal, QC, Canada (A01-E02-18A).
Figures







References
-
- Nozaki K, Saleh OIM, Arakawa S, Miura H. “Novel technologies to prevent dental plaque and calculus.” In Water-Formed Deposits. Elsevier; 2022. pp. 543–563.
-
- White DJ. Processes contributing to the formation of dental calculus. Biofouling. 1991;4:209–18.
-
- Karaaslan F, Demir T, Barış O. Effect of periodontal disease-associated bacteria on the formation of dental calculus: an in vitro study. J Adv Oral Res. 2020;11:165–71.
-
- Little JW. Complementary and alternative medicine: impact on dentistry. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2004;98:137–45. - PubMed
-
- Jepsen S, Deschner J, Braun A, Schwarz F, Eberhard J. Calculus removal and the prevention of its formation. Periodontology. 2011;55:167–88. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

