Safety, tolerability, and acceptability of long-acting injectable cabotegravir for HIV prevention in cisgender female adolescents (HPTN 084-01): a single-arm, open-label, phase 2b trial
- PMID: 40088909
- PMCID: PMC11961543
- DOI: 10.1016/S2352-3018(24)00310-2
Safety, tolerability, and acceptability of long-acting injectable cabotegravir for HIV prevention in cisgender female adolescents (HPTN 084-01): a single-arm, open-label, phase 2b trial
Abstract
Background: Long-acting formulations of HIV pre-exposure prophylaxis (PrEP) appear particularly well suited to adolescents. We aimed to establish the safety, tolerability, and acceptability of long-acting injectable cabotegravir as PrEP in cisgender adolescent girls.
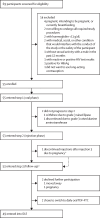
Methods: HPTN 084-01 is a single-arm, open-label, phase 2b trial conducted at three clinical research sites in South Africa, Uganda, and Zimbabwe. Girls were recruited via community study-outreach teams, reproductive health clinics, and peer referral. Sexually active adolescent girls (younger than 18 years) willing to use long-acting contraception, weighing at least 35 kg, and able to participate with parental or guardian consent (unless an emancipated minor) were eligible. After an oral lead-in, if no adverse events occurred, participants received a 3 mL intramuscular gluteal injection (long-acting injectable cabotegravir 600 mg) at weeks 5, 9, 17, 25, and 33. The product was discontinued for grade 3 or higher toxic effects or pregnancy. The primary outcomes were safety, tolerability, and acceptability. Safety (ie, proportions of grade 2 or higher clinical and laboratory events) was assessed at weeks 6, 10, 18, 26, and 34 in all enrolled participants. Injection tolerability (ie, proportions of premature discontinuation due to intolerability, frequency of injections, or burden of study procedures) and product acceptability (ie, proportions of scheduled injections completed and participants preferring long-acting injectable cabotegravir for future use) were assessed in all participants who received at least one injection at study end. The trial was registered with ClinicalTrials.gov (NCT04824131) and is completed.
Findings: Between Nov 1, 2020, and Aug 31, 2021, 69 participants were assessed for eligibility and 55 met inclusion criteria. The mean age was 16·0 years (SD 1·1), 39 (71%) had a recent primary sexual partner, 12 (22%) reported transactional sex, and 22 (40%) had sexually transmitted infections at baseline. Two participants dropped out and did not initiate long-acting injectable cabotegravir due to adverse events unrelated to the study drug during the oral lead-in. One participant stopped long-acting injectable cabotegravir after three injections due to pregnancy. 51 (93%) participants reported at least one adverse event of grade 2 or higher, mostly unrelated, transient laboratory abnormalities. There were no long-acting injectable cabotegravir discontinuations due to intolerability. Of the 52 participants who completed step 2, all scheduled injections were completed and 32 (62%) participants reported they would consider using long-acting injectable cabotegravir for HIV prevention in the future.
Interpretation: Long-acting injectable cabotegravir is a safe, tolerable, and acceptable option for the prevention of HIV in adolescent girls. Our study findings expand the HIV prevention options available to adolescent girls.
Funding: National Institute of Allergy and Infectious Diseases, National Institute of Mental Health, National Institute on Drug Abuse, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, ViiV Healthcare, and The Bill & Melinda Gates Foundation.
Copyright © 2025 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests CM is an employee of ViiV Healthcare. All other authors declare no competing interests.
Figures

References
-
- UNAIDS . Joint United Nations Programme on HIV/AIDS; 2024. The urgency of now: AIDS at a crossroads.
-
- WHO . World Health Organisation; 2022. Guidelines on long-acting injectable cabotegravir for HIV prevention. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

