Association between HIV-1 Nef-mediated MHC-I downregulation and the maintenance of the replication-competent latent viral reservoir in individuals with virally suppressed HIV-1 in Uganda: an exploratory cohort study
- PMID: 40088911
- PMCID: PMC12066219
- DOI: 10.1016/j.lanmic.2024.101018
Association between HIV-1 Nef-mediated MHC-I downregulation and the maintenance of the replication-competent latent viral reservoir in individuals with virally suppressed HIV-1 in Uganda: an exploratory cohort study
Abstract
Background: The persistence of a replication-competent latent viral reservoir (RC-LVR) during antiretroviral therapy (ART) is a barrier to the development of a cure for HIV-1, but the role of viral genes in influencing RC-LVR size is unclear. We aimed to assess whether the magnitude by which the HIV-1 accessory protein Nef evades the adaptive immune response by downregulating MHC-I or CD4, or both, from the surface of infected cells is associated with the rate at which the RC-LVR in people with HIV-1 changes during long-term ART (>1 year).
Methods: We conducted an exploratory cohort study in which nef genes were sequenced from outgrowth viruses derived from the quantitative viral outgrowth assay (QVOA) for a group of people with ART-suppressed HIV-1 in Uganda between 2015 and 2020. Study participants were selected from the Rakai Health Sciences Program (RHSP) LVR cohort, a cohort of 90 adults (aged ≥18 years) who were HIV-1 positive, receiving ART, and had maintained viral suppression for at least 1 year at the time of study enrolment. For this study, participants were required to have available p24+ QVOA wells that contained a single viral outgrowth isolate, as assessed by next-generation sequencing. In cases where further sequencing identified wells containing multiple viral clones, all sequenced nef variants were included for functional analysis. The unique isolated nef variants were used to generate pseudoviruses, which were employed to measure cell surface CD4 and MHC-I downregulation in infected CD4+ Sup-T1 cells via flow cytometry. The size and rate of change of the RC-LVR in participants was estimated using previous QVOA results and a Bayesian model. We then assessed whether a correlation existed between the extent to which the Nef proteins downregulated cell surface MHC-I and CD4 and the calculated RC-LVR rate of change during the study period.
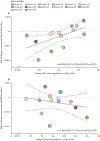
Findings: 14 (15%) of 90 participants from the RHSP cohort met the inclusion criteria and were enrolled in this study. 49 nef sequences were isolated from these participants. We observed variability in participant-derived Nef-mediated cell surface MHC-I downregulation (median 114·88% [IQR 104·93-121·51] of the downregulation capacity of NL4-3 Nef) and CD4 downregulation (94·50% [84·05-100·16] of NL4-3 Nef). The estimated rate of change of the RC-LVR was positive for four participants. For one donor, the rate of change was significantly positive (7·4 × 10-4 logit infectious units per million [IUPM] per day [95% credibility interval 3·2 × 10-4 to 1·2 × 10-3]) over the course of the study period (2015-20). The estimated rate of change of the RC-LVR for the remaining ten participants was negative, and significantly negative in four donors (-1·1 × 10-3 logit IUPM per day [95% credibility interval -1·8 × 10-3 to -3·7 × 10-4]; -1·4 × 10-3 [-2·0 × 10-3 to -8·5 × 10-4]; -7·0 × 10-4 [-1·3 × 10-3 to -1·6 × 10-4]; and -2·0 × 10-3 [-2·9 × 10-3 to -1·1 × 10-3]). A significant relationship between Nef-mediated MHC-I downregulation and the RC-LVR rate of change during the 5-year study period (r=0·6088 [95% CI 0·2366 to 0·9810]; p=0·023) was found, in which less efficient MHC-I downregulation correlated with faster RC-LVR decay during long-term ART. By contrast, Nef-mediated CD4 downregulation was not associated with RC-LVR rate of change during the 5-year study period (-0·1604 [-0·7311 to 0·4102]; p=0·58).
Interpretation: Nef-mediated MHC-I downregulation might contribute to HIV-1 persistence during long-term ART. Strategies to inhibit Nef-mediated MHC-I downregulation could represent a viable therapeutic avenue to reduce the size of the latent reservoir in vivo, improving treatment outcomes in people with HIV-1.
Funding: Canadian Institutes of Health Research, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, and the REACH Martin Delaney Collaboratory.
Copyright © 2024 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests We declare no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

