Ca2+-Triggered (de)ubiquitination Events in Synapses
- PMID: 40089065
- PMCID: PMC12008530
- DOI: 10.1016/j.mcpro.2025.100946
Ca2+-Triggered (de)ubiquitination Events in Synapses
Abstract
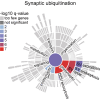
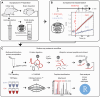
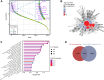
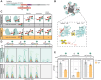
Neuronal communication relies on neurotransmitter release from synaptic vesicles (SVs), whose dynamics are controlled by Ca2+-dependent pathways, as many thoroughly studied phosphorylation cascades. However, little is known about other post-translational modifications, such as ubiquitination. To address this, we analyzed resting and stimulated synaptosomes (isolated synapses) by quantitative mass spectrometry. We identified more than 5000 ubiquitination sites on ∼2000 proteins, the majority of which participate in SV recycling processes. Several proteins showed significant changes in ubiquitination in response to Ca2+ influx, with the most pronounced changes in CaMKIIα and the clathrin adaptor protein AP180. To validate this finding, we generated a CaMKIIα mutant lacking the ubiquitination target site (K291) and analyzed it both in neurons and non-neuronal cells. K291 ubiquitination, close to an important site for CaMKIIα autophosphorylation (T286), influences the synaptic function of this kinase. We suggest that ubiquitination in response to synaptic activity is an important regulator of synaptic function.
Keywords: CAMKI; calcium; post-translational modification; synapse; ubiquitin.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors state that they have no conflicts of interest with the contents of the article.
Figures


References
-
- Toonen R.F.G., Verhage M. Munc18-1 in secretion: lonely Munc joins SNARE team and takes control. Trends Neurosci. 2007;30:564–572. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

