Metabolic Reprogramming Into a Glycolysis Phenotype Induced by Extracellular Vesicles Derived From Prostate Cancer Cells
- PMID: 40089067
- PMCID: PMC12008616
- DOI: 10.1016/j.mcpro.2025.100944
Metabolic Reprogramming Into a Glycolysis Phenotype Induced by Extracellular Vesicles Derived From Prostate Cancer Cells
Abstract
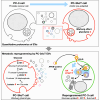
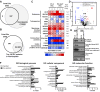
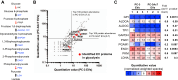
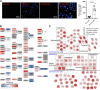
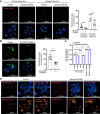
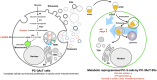
Most cancer cells adopt a less efficient metabolic process of aerobic glycolysis with high level of glucose uptake followed by lactic acid production, known as the Warburg effect. This phenotypic transition enables cancer cells to achieve increased cellular survival and proliferation in a harsh low-oxygen tumor microenvironment. Also, the resulting acidic microenvironment causes inactivation of the immune system such as T-cell impairment that favors escape by immune surveillance. While lots of studies have revealed that tumor-derived EVs can deliver parental materials to adjacent cells and contribute to oncogenic reprogramming, their functionality in energy metabolism is not well addressed. In this study, we established prostate cancer cells PC-3AcT resistant to cellular death in an acidic culture medium driven by lactic acid. Quantitative proteomics between EVs derived from PC-3 and PC-3AcT cells identified 935 confident EV proteins. According to cellular adaptation to lactic acidosis, we revealed 159 regulated EV proteins related to energy metabolism, cellular shape, and extracellular matrix. These EVs contained a high abundance of glycolytic enzymes. In particular, PC-3AcT EVs were enriched with apolipoproteins including apolipoprotein B-100 (APOB). APOB on PC-3AcT EVs could facilitate their endocytic uptake depending on low density lipoprotein receptor of recipient PC-3 cells, encouraging increases of cellular proliferation and survival in acidic culture media via increased activity and expression of hexokinases and phosphofructokinase. The activation of recipient PC-3 cells can increase glucose consumption and ATP generation, representing an acquired metabolic reprogramming into the Warburg phenotype. Our study first revealed that EVs derived from prostate cancer cells could contribute to energy metabolic reprogramming and that the acquired metabolic phenotypic transition of recipient cells could favor cellular survival in tumor microenvironment.
Keywords: cancer; exosomes; extracellular vesicles; metabolism; proteomics.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest The authors declare no competing interests.
Figures



References
-
- Lehuede C., Dupuy F., Rabinovitch R., Jones R.G., Siegel P.M. Metabolic plasticity as a determinant of tumor growth and metastasis. Cancer Res. 2016;76:5201–5208. - PubMed
-
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. - PubMed
-
- Pfeiffer T., Schuster S., Bonhoeffer S. Cooperation and competition in the evolution of ATP-producing pathways. Science. 2001;292:504–507. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

