Chronic administration of ivabradine improves cardiac Ca handling and function in a rat model of Duchenne muscular dystrophy
- PMID: 40089543
- PMCID: PMC11910634
- DOI: 10.1038/s41598-025-92927-4
Chronic administration of ivabradine improves cardiac Ca handling and function in a rat model of Duchenne muscular dystrophy
Abstract
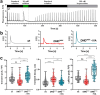
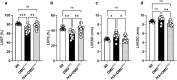
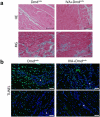
Duchenne muscular dystrophy (DMD), a severe muscle disease caused by mutations in the gene encoding for the intracellular protein dystrophin, is associated with impaired cardiac function and arrhythmias. A causative factor for complications in the dystrophic heart is abnormal calcium (Ca) handling in ventricular cardiomyocytes, and restoration of normal Ca homeostasis has emerged as therapeutic strategy. Here, we used a rodent model of DMD, the dystrophin-deficient DMDmdx rat, to test the following hypothesis: chronic administration of ivabradine (IVA), a drug clinically approved for the treatment of heart failure, improves Ca handling in dystrophic ventricular cardiomyocytes and thereby enhances contractile performance in the dystrophic heart. Intracellular Ca measurements revealed that 4-months administration of IVA to DMDmdx rats significantly improves Ca handling properties in dystrophic ventricular cardiomyocytes. In particular, IVA treatment increased electrically-evoked Ca transients and speeded their decay. This suggested enhanced sarcoplasmic reticulum Ca release and faster removal of Ca from the cytosol. Chronic IVA administration also enhanced the sarcoplasmic reticulum Ca load. Transthoracic echocardiography revealed a significant improvement of cardiac systolic function in IVA-treated DMDmdx rats. Thus, left ventricular ejection fraction and fractional shortening were enhanced, and end-systolic as well as end-diastolic diameters were diminished by the drug. Finally, chronic IVA administration neither significantly attenuated cardiac fibrosis and apoptosis, nor was vascular function improved by the drug. Collectively our findings suggest that long-term IVA administration enhances contractile function in the dystrophic heart by improvement of Ca handling in ventricular cardiomyocytes. Chronic IVA administration may be beneficial for DMD patients.
Keywords: Cardiac function; Cardiomyocyte calcium handling; Duchenne muscular dystrophy; Dystrophic rats; Ivabradine treatment.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The study is reported in accordance with ARRIVE guidelines. No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


References
-
- Koruth, J. S., Lala, A., Pinney, S., Reddy, V. Y. & Dukkipati, S. R. The clinical use of ivabradine. J. Am. Coll. Cardiol.70, 1777–1784 (2017). - PubMed
-
- Finsterer, J., Stöllberger, C. & Berger, E. Beneficial effect of ivabradine in dilated cardiomyopathy from Becker muscular dystrophy. Herz37, 702–705 (2012). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

