Shank3 modulates Rpl3 expression and protein synthesis via mGlu5: implications for Phelan McDermid syndrome
- PMID: 40089604
- PMCID: PMC12240844
- DOI: 10.1038/s41380-025-02947-9
Shank3 modulates Rpl3 expression and protein synthesis via mGlu5: implications for Phelan McDermid syndrome
Abstract
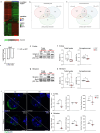
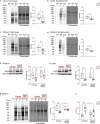
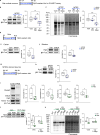
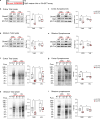
Mutations or deletions in the SHANK3 gene have been identified in up to 1% of autism spectrum disorder cases and are considered the primary cause of neuropsychiatric symptoms in Phelan McDermid syndrome (PMS). While synaptic dysfunctions have been extensively documented in the absence of Shank3, other mechanisms through which Shank3 may regulate neuronal functions remain unclear. In this study, we report that the ribosomal protein Rpl3 and overall protein synthesis are downregulated in the cortex and striatum of Shank3 knockout (KO) mice and in neurons differentiated from human-induced pluripotent stem cells (hiPSCs) derived from a PMS patient. Moreover, restoring Rpl3 expression in the striatum of Shank3 KO mice was sufficient to rescue protein synthesis and mitigate excessive grooming, suggesting that the behavioral alterations observed in Shank3 KO mice might be, at least in part, caused by Rpl3 downregulation and consequent impaired protein synthesis. Furthermore, we demonstrated that chronic inhibition of mGlu5 is sufficient to reduce Rpl3 expression, which in turn impairs global protein synthesis. Consequently, chronic treatment with VU0409551, a potent and selective mGlu5 positive allosteric modulator, rescues Rpl3 expression and the resulting reduction in protein synthesis, leading to long-lasting improvements in behavioral deficits in Shank3 KO mice Altogether, we propose a new role for Shank3 in modulating Rpl3 protein expression, ribosomal function, and protein synthesis by downregulating mGlu5 receptor activity.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interest. Ethics approval: All methods in this study were performed in accordance with the relevant guidelines and regulations. All animal procedures were performed in accordance with the relevant guidelines and regulations and were approved by the Italian Ministry of Health, Authorization number: 966/2016-PR and 103-2024-PR.
Figures

References
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials

