The LINC Complex Regulates Tendon Elastic Modulus, Collagen Crimp, and Lateral Expansion During Early Postnatal Development
- PMID: 40089904
- PMCID: PMC12129308
- DOI: 10.1002/jor.26069
The LINC Complex Regulates Tendon Elastic Modulus, Collagen Crimp, and Lateral Expansion During Early Postnatal Development
Abstract
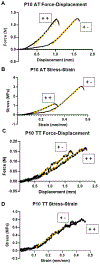
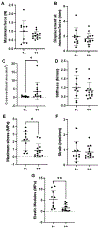
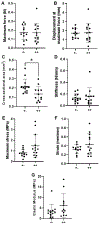
There is limited understanding of how mechanical signals regulate tendon development. The nucleus has emerged as a major regulator of cellular mechanosensation via the linker of nucleoskeleton and cytoskeleton (LINC) protein complex. Specific roles of LINC in tenogenesis have not been explored. In this study, we investigate how LINC regulates tendon development by disabling LINC-mediated mechanosensing via dominant negative (dn) overexpression of the Klarsicht, ANC-1, and Syne Homology (KASH) domain, which is necessary for LINC to function. We hypothesized that LINC regulates mechanotransduction in developing tendons and that disabling LINC would impact tendon's mechanical properties and structure in a mouse model of dnKASH. We used Achilles tendon (AT) and tail tendon (TT) as representative energy-storing and positional tendons, respectively. Mechanical testing at postnatal day 10 showed that disabling the LINC complex via dnKASH significantly impacted tendon mechanical properties and cross-sectional area and that the effects differed between ATs and TTs. Collagen crimp distance was also impacted in dnKASH tendons and was significantly decreased in ATs and increased in TTs. Overall, we show that disruption to the LINC complex specifically impacts tendon mechanics and collagen crimp structure, with unique responses between an energy-storing and limb-positioning tendon. This suggests that nuclear mechanotransduction through LINC plays a role in regulating tendon formation during neonatal development.
Keywords: KASH‐domain; LINC; SUN‐domain; development; mechanobiology; mechanotransduction; nesprin; nuclear mechanosensing; tendon; tenogenesis.
© 2025 Orthopaedic Research Society.
Conflict of interest statement
Conflicts of Interest
The authors have no conflicts of interest to declare.
Figures
Update of
-
The LINC complex regulates Achilles tendon elastic modulus, Achilles and tail tendon collagen crimp, and Achilles and tail tendon lateral expansion during early postnatal development.bioRxiv [Preprint]. 2023 Nov 13:2023.11.13.566892. doi: 10.1101/2023.11.13.566892. bioRxiv. 2023. Update in: J Orthop Res. 2025 Jun;43(6):1090-1100. doi: 10.1002/jor.26069. PMID: 38014288 Free PMC article. Updated. Preprint.
Similar articles
-
The LINC complex regulates Achilles tendon elastic modulus, Achilles and tail tendon collagen crimp, and Achilles and tail tendon lateral expansion during early postnatal development.bioRxiv [Preprint]. 2023 Nov 13:2023.11.13.566892. doi: 10.1101/2023.11.13.566892. bioRxiv. 2023. Update in: J Orthop Res. 2025 Jun;43(6):1090-1100. doi: 10.1002/jor.26069. PMID: 38014288 Free PMC article. Updated. Preprint.
-
Depletion of SUN1/2 induces heterochromatin accrual in mesenchymal stem cells during adipogenesis.Commun Biol. 2025 Mar 13;8(1):428. doi: 10.1038/s42003-025-07832-3. Commun Biol. 2025. PMID: 40082539 Free PMC article.
-
Early (< 8 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants.Cochrane Database Syst Rev. 2017 Oct 24;10(10):CD001146. doi: 10.1002/14651858.CD001146.pub5. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2021 Oct 21;10:CD001146. doi: 10.1002/14651858.CD001146.pub6. PMID: 29063585 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
References
-
- Arampatzis A, Karamanidis K, and Albracht K. Adaptational responses of the human Achilles tendon by modulation of the applied cyclic strain magnitude. J. Exp. Biol 210:2743–2753, 2007. - PubMed
-
- Banes AJ, Horesovsky G, Larson C, Tsuzaki M, Judex S, Archambault J, Zernicke R, Herzog W, Kelley S, and Miller L. Mechanical load stimulates expression of novel genes in vivo and in vitro in avian flexor tendon cells. Osteoarthritis Cartilage 7:141–53, 1999. - PubMed
-
- Birks S, Howard S, O’Rourke C, Thompson WR, Lau A, and Uzer G. Osterix-driven LINC complex disruption in vivo diminishes bone microarchitecture in 8-week male mice but not after 6-week voluntary wheel running. BioRxiv Prepr. Serv. Biol. 2023.08.24.554623, 2023.doi: 10.1101/2023.08.24.554623 - DOI
MeSH terms
Substances
Grants and funding
- C06 RR020533/RR/NCRR NIH HHS/United States
- P20 GM103408/GM/NIGMS NIH HHS/United States
- P20 GM109095/GM/NIGMS NIH HHS/United States
- This project was made possible by a NASA EPSCoR Research Initiation Grant and a Beckman Scholars Award from the Arnold and Mabel Beckman Foundation (to N.M.P.). We acknowledge support from the Institutional Development Awards (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under Grants #P20GM103408, P20GM109095 P20GM109095, AG059923, AR075803, and 1C06RR020533 and from the National Science Foundation under Grants 1929188 and 2025505. We also acknowledge support from The Biomolecular Research Center at Boise State, BSU-Biomolecular Research Center, RRID:SCR_019174, with funding from the National Science Foundation, Grants #0619793 and #0923535; the M. J. Murdock Charitable Trust; Lori and Duane Stueckle; and the Idaho State Board of Education.
- R01 AG059923/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources

