Blunted CD40-responsive enhancer activation in CREBBP-mutant lymphomas can be restored by enforced CD4 T-cell engagement
- PMID: 40090010
- PMCID: PMC12333230
- DOI: 10.1182/blood.2024026664
Blunted CD40-responsive enhancer activation in CREBBP-mutant lymphomas can be restored by enforced CD4 T-cell engagement
Abstract
The CREBBP lysine acetyltransferase (KAT) is frequently mutated in follicular lymphoma and diffuse large B-cell lymphoma and has been studied using gene knockout in murine and human cells. However, most CREBBP mutations encode amino acid substitutions within the catalytic KAT domain (CREBBP KAT-PM) that retain an inactive protein and have not been extensively characterized. Using CRISPR gene editing and extensive epigenomic characterization of lymphoma cell lines, we found that CREBBP KAT-PM lead to unloading of CREBBP from chromatin, loss of enhancer acetylation, and prevention of EP300 compensation. These enhancers were enriched for those that are dynamically loaded by CREBBP in the normal centroblast-to-centrocyte transition in the germinal center, including enhancers activated in response to CD40 signaling, leading to blunted molecular response to CD40 ligand in lymphoma cells. We provide evidence that CREBBP KAT-PM inhibits EP300 function by binding limiting quantities nuclear transcription factor (TF), thereby preventing its compensatory activity. This effect can be experimentally overcome by expressing saturating quantities of TF or biologically attenuated by strong stimulation of CD40 signaling that increases nuclear TF abundance. Importantly, epigenetic responses to CD40 signaling can be induced by enforcing CD4 T-cell engagement using a bispecific antibody, leading to CD40-dependent restoration of antigen presentation machinery in CREBBP KAT-PM cells and cell death. Therefore, we provide a mechanistic basis for enhancer deregulation by CREBBP KAT-PM and highlight enforced CD4 T-cell engagement as a potential approach for overcoming these effects.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: M.R.G. reports research funding from Sanofi (including this study), Kite/Gilead, AbbVie, and Allogene; consulting for AbbVie, Allogene, and Bristol Myers Squibb; honoraria from Daiichi Sankyo and DAVA Oncology; and stock ownership of KDAc Therapeutics. S.N. received research support from Kite/Gilead, Bristol Myers Squibb, Cellectis, Poseida, Allogene, Unum Therapeutics, Precision Biosciences, and Adicet Bio; served as advisory board member/consultant for Kite/Gilead, Merck, Novartis, SELLAS Life Sciences, Athenex, Allogene, Incyte, Adicet Bio, Bristol Myers Squibb, Legend Biotech, bluebird bio, Fosun Kite, Sana Biotechnology, Caribou, Astellas Pharma, MorphoSys, Janssen, Chimagen, ImmunoACT, and Orna Therapeutics; has received royalty income from Takeda Pharmaceuticals; has stock options from Longbow Immunotherapy, Inc; and has intellectual property related to cell therapy. L.N. reports honoraria for participation on advisory boards from ADC Therapeutics, Atara, Bristol Myers Squibb, Caribou Biosciences, Epizyme, Genentech, Genmab, Gilead/Kite, Janssen, MorphoSys, Novartis, and Takeda; reports research support from Bristol Myers Squibb, Caribou Biosciences, Epizyme, Genentech, Genmab, Gilead/Kite, Janssen, IGM Biosciences, Novartis, and Takeda; and serves on a data safety and monitoring board for Denovo, Genentech, MEI Pharma, and Takeda. C.R.F. reports consulting for AbbVie, Bayer, BeiGene, Celgene, Denovo Biopharma, Foresight Diagnostics, Genentech/Roche, Genmab, Gilead, Karyopharm, N-Power Medicine, Pharmacyclics/Janssen, Seagen, and Spectrum; research funding from 4D, AbbVie, Acerta, Adaptimmune, Allogene, Amgen, Bayer, Celgene, Cellectis EMD, Gilead, Genentech/Roche, Guardant, Iovance, Janssen Pharmaceuticals, Kite, MorphoSys, Nektar, Novartis, Pfizer, Pharmacyclics, Sanofi, Takeda, TG Therapeutics, Xencor, Ziopharm, Burroughs Wellcome Fund, Eastern Cooperative Oncology Group, National Cancer Institute, V Foundation, and Cancer Prevention and Research Institute of Texas; and stock options in Foresight Diagnostics and N-Power Medicine. The remaining authors declare no competing financial interests.
Figures

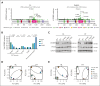
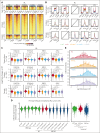
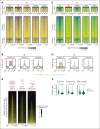

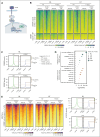



Comment in
-
Beware the zombie enzyme.Blood. 2025 Jul 10;146(2):135-136. doi: 10.1182/blood.2025028953. Blood. 2025. PMID: 40638202 No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

