Mortality Risk Among Patients With Influenza Illness Admitted to the ICU: A Systematic Review and Meta-Analysis
- PMID: 40090895
- PMCID: PMC11911131
- DOI: 10.1111/irv.70073
Mortality Risk Among Patients With Influenza Illness Admitted to the ICU: A Systematic Review and Meta-Analysis
Abstract
Background: Despite vaccination programs and available treatments, seasonal influenza carries a large mortality burden, especially in intensive care unit (ICU) settings. Understanding the influenza mortality burden in ICU settings can inform treatment planning and resource allocation. Nonetheless, surveillance data on mortality in ICU-admitted patients are scarce and estimates vary greatly. This systematic literature review (SLR) and meta-analysis investigated all-cause mortality risk among ICU-admitted patients with influenza in Europe.
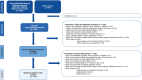
Methods: We included observational studies conducted in Europe that reported mortality among patients ≥ 6 months of age with influenza admitted to the ICU. Studies published between January-2009 and December-2019 were included. Quality was assessed using a modified Newcastle-Ottawa scale. Pooled all-cause mortality risk was calculated as a proportion using a random-effects model with an inverse variance method. A sensitivity analysis was also conducted, including only studies identified as having low risk of bias.
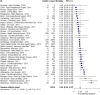
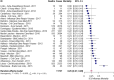
Results: Thirty-seven studies, reporting on 13,616 patients, were included. All-cause mortality ranged from 0% to 67%. The overall pooled mortality risk estimate was 0.24 (95% CI: 0.20, 0.27). Study heterogeneity was high (Cochran's Q test p < 0.01, I2 = 93%). The sensitivity analysis using only studies identified as having low risk of bias produced a pooled mortality risk of 0.25 (95%CI: 0.21, 0.29).
Conclusions: These results indicate that approximately a quarter of patients with influenza admitted to the ICU die, reinforcing the need for effective vaccination programs and treatment optimization.
Keywords: influenza; intensive care unit; meta‐analysis; mortality; neuraminidase inhibitors.
© 2025 GSK plc. Influenza and Other Respiratory Viruses published by John Wiley & Sons Ltd.
Conflict of interest statement
Pablo Suarez‐Sanchez, Jara Majuelos, Marina Hinojosa‐Campos, Bélène Podmore are employees of OXON Epidemiology Ltd Epidemiology & Statistics, Madrid, Spain, an independent contract research organization, which received funding from GSK to design and conduct this study. Iain A Gillespie, Jennifer Han, Rosa Sloot, and Dina Christensen are employees of and may hold shares in the GSK group of companies.
Figures
References
-
- World Health Organization , “Influenza,” (2023), accessed 1 June 2023, https://www.who.int/teams/health‐product‐policy‐and‐standards/standards‐....
-
- European Centre for Disease Prevention and Control , “Disease facts About Seasonal Influenza,” (2023), accessed 30 Aug 2023, https://www.ecdc.europa.eu/en/seasonal‐influenza/facts.
-
- Fiore A., Fry A., Shay D., Gubareva L., Bresee J. S., and Uyeki T. M., “Antiviral Agents for the Treatment and Chemoprophylaxis of Influenza–Recommendations of the Advisory Committee on Immunization Practices (ACIP),” MMWR Recommendations and Reports: Morbidity and Mortality Weekly Report Recommendations and Reports 60, no. 1 (2011): 1–24. - PubMed
-
- UK Health Security Agency . Guidance on Use of Antiviral Agents for the Treatment and Prophylaxis of Seasonal Influenza 2019.
-
- Pernille J., Jolita M., Suzanne C., Kari J., Svetla T., and Caroline B., “How Close Are Countries of the WHO European Region to Achieving the Goal of Vaccinating 75% of Key Risk Groups Against Influenza? Results From National Surveys on Seasonal Influenza Vaccination Programmes, 2008/2009 to 2014/2015,” Vaccine 36, no. 4 (2018): 442–452. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical