CCL20/CXCL5 Drives Crosstalk Between Anaplastic Thyroid Cancer Stem Cells and Tumor-Associated Macrophages to Promote Tumor Progression
- PMID: 40091357
- PMCID: PMC12061268
- DOI: 10.1002/advs.202405399
CCL20/CXCL5 Drives Crosstalk Between Anaplastic Thyroid Cancer Stem Cells and Tumor-Associated Macrophages to Promote Tumor Progression
Abstract
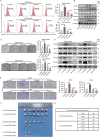
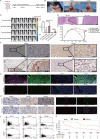
The dynamic interplay between tumor-associated macrophages (TAMs) and anaplastic thyroid cancer (ATC) shapes the tumor microenvironment and facilitates ATC progression. However, the mechanisms of communication between TAMs and anaplastic thyroid cancer stem cells (ATCSCs) remain largely unelucidated. Integrative analyses of single-cell RNA sequencing, cytokine/chemokine arrays, proteomics, and mRNA expression datasets are performed to reveal crosstalk between TAMs and ATCSCs and signaling pathways in ATCSCs. Subsequently, in vitro experiments are performed to validate the regulatory effects of key cytokines on ATCSC stemness. Last, xenogeneic orthotopic thyroid ATCSCs transplantation models are utilized to corroborate the regulatory effect of cytokines on stemness. CCL20 derived from THP-1-M2 activates the IRAK-1/NF-κB1/2 signaling pathway in ATCSCs, thereby positively regulating stemness characteristics and upregulating CXCL5 secretion. ATCSCs not only exhibit autocrine CXCL5 participation in the regulation of stemness but also demonstrate paracrine CXCL5 activity to recruit THP-1-Mφ and maintain the M2 phenotype. CCL20 and CXCL5 are involved in the crosstalk between TAMs and ATCSCs. The CCL20/CXCL5 axis plays a crucial role in the interaction between TAMs and ATCSCs, establishing a progressive tumor microenvironment.
Keywords: CCL20; CXCL5; anaplastic thyroid cancer; cancer stem cell; crosstalk; tumor microenvironment.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Bible K. C., Kebebew E., Brierley J., Brito J. P., Cabanillas M. E., Clark T. J. Jr., Di Cristofano A., Foote R., Giordano T., Kasperbauer J., Newbold K., Nikiforov Y. E., Randolph G., Rosenthal M. S., Sawka A. M., Shah M., Shaha A., Smallridge R., Wong‐Clark C. K., Thyroid 2021, 31, 337. - PMC - PubMed
-
- Smallridge R. C., Ain K. B., Asa S. L., Bible K. C., Brierley J. D., Burman K. D., Kebebew E., Lee N. Y., Nikiforov Y. E., Rosenthal M. S., Shah M. H., Shaha A. R., Tuttle R. M., Thyroid 2012, 22, 1104. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
