IL-33-Induced TREM2+ Macrophages Promote Pathological New Bone Formation Through CREG1-IGF2R Axis in Ankylosing Spondylitis
- PMID: 40091508
- PMCID: PMC12079337
- DOI: 10.1002/advs.202500952
IL-33-Induced TREM2+ Macrophages Promote Pathological New Bone Formation Through CREG1-IGF2R Axis in Ankylosing Spondylitis
Abstract
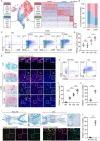
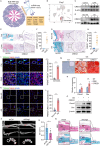
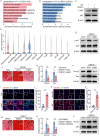
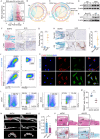
Pathological new bone formation is the main cause of disability in ankylosing spondylitis (AS), and so far, it lacks a targeted therapy. Macrophages are central orchestrators of inflammation progression and tissue remodeling, but their contribution to pathological new bone formation has largely not been explored. Here, it is identified that TREM2+ macrophages predominated within the sites of new bone formation and adjacent to osteogenic precursor cells. In vivo, both depletion of macrophages and knockout of Trem2 significantly reduced pathological new bone formation in a collagen antibody-induced arthritis (CAIA) model. Specifically, TREM2+ macrophages promoted osteogenic differentiation of ligament-derived progenitor cells (LDPCs) by secreting CREG1, a secretory glycoprotein involved in cell differentiation and normal physiology. CREG1-IGF2R-PI3K-AKT signaling pathway is involved in TREM2+ macrophage-mediated pathological new bone formation. In addition, it is found that IL-33 promoted TREM2+ macrophage differentiation through phosphorylation of STAT6. Targeting the above signalings alleviated new bone formation in the CAIA model. The findings highlight the critical role of IL-33-induced TREM2+ macrophages in pathological new bone formation and provide potential therapeutic targets for halting spinal ankylosis in AS.
Keywords: CREG1; IL33; TREM2+ macrophages; ankylosing spondylitis; pathological new bone formation.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Sieper J., Poddubnyy D., Lancet 2017, 390, 73. - PubMed
-
- Navarro‐Compan V., Sepriano A., El‐Zorkany B., van der Heijde D., Ann. Rheum. Dis. 2021, 80, 1511. - PubMed
-
- Malinowski K. P., Kawalec P., Expert Rev. Pharmacoecon. Outcomes Res. 2015, 15, 285. - PubMed
-
- Lories R., Nat. Rev. Rheumatol. 2011, 7, 700. - PubMed
-
- Ward M. M., Deodhar A., Gensler L. S., Dubreuil M., Yu D., Khan M. A., Haroon N., Borenstein D., Wang R., Biehl A., Fang M. A., Louie G., Majithia V., Ng B., Bigham R., Pianin M., Shah A. A., Sullivan N., Turgunbaev M., Oristaglio J., Turner A., Maksymowych W. P., Caplan L., Arthritis Care Res. 2019, 71, 1285. - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 82172384/National Natural Science Foundation of China
- 81972039/National Natural Science Foundation of China
- 82372370/National Natural Science Foundation of China
- 2021B1515020080/Department of Science and Technology of Guangdong Province
- 22yklj02/Innovation Talent Cultivation Program of Sun Yat-sen University
- Y12001/KELIN New Talent Project of The First Affiliated Hospital, Sun Yat-sen University
- 2022TFC2502901/National Key R&D Program of China
- 2022YFC2502902/National Key R&D Program of China
- 2022YFC2502903/National Key R&D Program of China
- 2022YFC2502904/National Key R&D Program of China
- 2021B1212060002/Guangdong Provincial Platform Base and Science and Technology Infrastructure Construction Project
- 2023B03J1257/The Key Scientific and Technological Program of Guangzhou City
- BX20240433/Fellowship of China National Postdoctoral Program for Innovative Talents
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
