EFNA2 Mediates Stiffness-Regulated Hypopharyngeal Cancer Progression
- PMID: 40091591
- PMCID: PMC12146608
- DOI: 10.21053/ceo.2024.00315
EFNA2 Mediates Stiffness-Regulated Hypopharyngeal Cancer Progression
Abstract
Objectives: Hypopharyngeal cancer (HPC), originating from the hypopharyngeal mucosa, is associated with a poor prognosis. Extracellular matrix (ECM) stiffness is closely linked to tumor progression and patient outcomes. This study aimed to investigate the effects of matrix stiffness and to identify molecular markers relevant to HPC prognosis, with the goal of improving clinical outcomes.
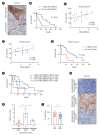
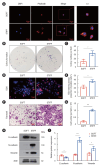
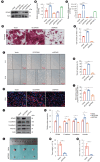
Methods: Immunohistochemical analysis and cervical enhanced computed tomography (CT) data were used to evaluate correlations among CT values, matrix stiffness, and prognosis, and to develop a prognostic model for HPC. Cell culture models with varying matrix stiffness were established using polypropylene hydrogel. Western blotting, colony formation, EDU incorporation, and Transwell assays were employed to assess the effects of matrix stiffness on proliferation, migration, invasion, and epithelial-mesenchymal transition (EMT). High-throughput sequencing identified differentially expressed genes in HPC cells cultured on matrices of differing stiffness. Gene editing, in vivo subcutaneous tumorigenesis studies, and Western blotting were performed to elucidate the molecular mechanisms by which high matrix stiffness influences HPC progression and the role of ephrin A2 (EFNA2) in proliferation, migration, and EMT.
Results: Arterial phase CT values were positively correlated with matrix stiffness. Increased matrix stiffness was associated with lymph node metastasis, diminished therapeutic response, and poorer prognosis. Furthermore, metastatic lymph nodes in HPC patients exhibited higher CT values than those in patients with nasopharyngeal carcinoma. A high-stiffness ECM promoted proliferation, migration, and EMT in HPC cells. Mechanistically, a stiff ECM enhanced EFNA2 expression, thereby promoting proliferation, migration, EMT, and tumor growth in vivo.
Conclusion: EFNA2 and elevated matrix stiffness jointly contribute to the malignant phenotype in HPC. EFNA2 may represent a potential therapeutic target for managing HPC progression induced by high matrix stiffness.
Keywords: EFNA2; Epithelial-Mesenchymal Transition; Hypopharyngeal Carcinoma; Matrix Stiffness.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures


Similar articles
-
PKM2 promotes lymphatic metastasis of hypopharyngeal carcinoma via regulating epithelial-mesenchymal transition: an experimental research.Diagn Pathol. 2024 Mar 2;19(1):48. doi: 10.1186/s13000-024-01474-5. Diagn Pathol. 2024. PMID: 38431604 Free PMC article.
-
Lentivirus-Mediated Short Hairpin RNA for Follistatin Downregulation Suppresses Tumor Progression in Hypopharyngeal Carcinoma.Curr Med Sci. 2022 Aug;42(4):832-840. doi: 10.1007/s11596-022-2615-3. Epub 2022 Jul 21. Curr Med Sci. 2022. PMID: 35864413
-
TEAD4 functions as a prognostic biomarker and triggers EMT via PI3K/AKT pathway in bladder cancer.J Exp Clin Cancer Res. 2022 May 17;41(1):175. doi: 10.1186/s13046-022-02377-3. J Exp Clin Cancer Res. 2022. PMID: 35581606 Free PMC article.
-
Biophysics Role and Biomimetic Culture Systems of ECM Stiffness in Cancer EMT.Glob Chall. 2022 Mar 20;6(6):2100094. doi: 10.1002/gch2.202100094. eCollection 2022 Jun. Glob Chall. 2022. PMID: 35712024 Free PMC article. Review.
-
SPOCK1 Involvement in Epithelial-to-Mesenchymal Transition: A New Target in Cancer Therapy?Cancer Manag Res. 2020 May 18;12:3561-3569. doi: 10.2147/CMAR.S249754. eCollection 2020. Cancer Manag Res. 2020. PMID: 32547193 Free PMC article. Review.
References
-
- Chow LQM. Head and neck cancer. N Engl J Med. 2020 Jan;382(1):60–72. - PubMed
-
- Kwon DI, Miles BA, Education Committee of the American Head and Neck Society (AHNS) Hypopharyngeal carcinoma: do you know your guidelines. Head Neck. 2019 Mar;41(3):569–76. - PubMed
-
- Wells RG. The role of matrix stiffness in regulating cell behavior. Hepatology. 2008 Apr;47(4):1394–400. - PubMed
-
- Feng J, Tang Y, Xu Y, Sun Q, Liao F, Han D. Substrate stiffness influences the outcome of antitumor drug screening in vitro. Clin Hemorheol Microcirc. 2013;55(1):121–31. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

