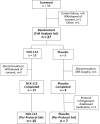
NLX-112 Randomized Phase 2A Trial: Safety, Tolerability, Anti-Dyskinetic, and Anti-Parkinsonian Efficacy
- PMID: 40091754
- PMCID: PMC12160962
- DOI: 10.1002/mds.30175
NLX-112 Randomized Phase 2A Trial: Safety, Tolerability, Anti-Dyskinetic, and Anti-Parkinsonian Efficacy
Abstract
Background: Levodopa-induced dyskinesia (LID) in Parkinson's disease (PD) is associated with 'false neurotransmitter' release of dopamine from serotonin (5-HT) neurons. NLX-112 is a first-in-class, highly selective 5-HT1A receptor agonist which counteracts LIDs in experimental PD models.
Objectives: The primary objective was to evaluate the safety and tolerability of NLX-112 compared with placebo in people with PD. The secondary objective was to assess the preliminary efficacy of NLX-112 in reducing LID and its effects on PD symptoms.
Methods: Participants received NLX-112 or placebo (2:1 ratio) alongside stable Parkinson's medications, with 22 participants completing the study. Dosing was up-titrated over 28 days to 2 mg/day (1 mg twice daily), stabilized for 14 days (to day 42), and down-titrated for 14 days. Efficacy was measured using the Unified Dyskinesia Rating Scale (UDysRS), Unified Parkinson's Disease Rating Scale (UPDRS), and Clinical Global Impression of Change (CGI-C) following a levodopa challenge (150% of usual dose).
Results: Adverse events (AEs) were mainly central nervous system (CNS)-related and mostly occurred during up-titration, with no serious AEs in the NLX-112 group. There were no treatment-induced clinically significant changes in vital signs, electrocardiogram, or laboratory parameters. NLX-112 reduced LID from baseline levels: at day 42, UDysRS total score decreased by 6.3 points, whereas placebo group changes were not significant (-2.4). NLX-112 also reduced parkinsonism from baseline values: UPDRS Part 3 scores decreased by 3.7 points, whereas placebo group changes were non-significant (+0.1). In CGI-C assessment, the NLX-112 group showed greater improvement than the placebo group (53% vs. 29%).
Conclusion: These results support further clinical investigation of NLX-112 for treatment of PD LID. © 2025 Neurolixis SAS. Movement Disorders published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society.
Keywords: 5‐HT1A receptor; NLX‐112; Parkinson's disease; dyskinesia; levodopa; serotonin.
© 2025 Neurolixis SAS. Movement Disorders published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society.
Figures
References
-
- Manson A, Stirpe P, Schrag A. Levodopa‐induced‐dyskinesias clinical features, incidence, risk factors, management and impact on quality of life. J Parkinsons Dis 2012;2(3):189–198. - PubMed
-
- PD Med Collaborative Group , Gray R, Ives N, et al. Long‐term effectiveness of dopamine agonists and monoamine oxidase B inhibitors compared with levodopa as initial treatment for Parkinson's disease (PD MED): a large, open‐label, pragmatic randomised trial. Lancet 2014;384(9949):1196–1205. 10.1016/S0140-6736(14)60683-8 - DOI - PubMed
-
- Maeda T, Nagata K, Yoshida Y, Kannari K. Serotonergic hyperinnervation into the dopaminergic denervated striatum compensates for dopamine conversion from exogenously administered l‐DOPA. Brain Res 2005;1046(1–2):230–233. - PubMed
-
- Arai R, Karasawa N, Nagatsu I. Aromatic L‐amino acid decarboxylase is present in serotonergic fibers of the striatum of the rat. A double‐labeling immunofluorescence study. Brain Res 1996;706(1):177–179. - PubMed
-
- Arai R, Karasawa N, Geffard M, Nagatsu T, Nagatsu I. Immunohistochemical evidence that central serotonin neurons produce dopamine from exogenous L‐DOPA in the rat, with reference to the involvement of aromatic L‐amino acid decarboxylase. Brain Res 1994;667(2):295–299. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical