Activating antiviral immune responses potentiates immune checkpoint inhibition in glioblastoma models
- PMID: 40091830
- PMCID: PMC11910234
- DOI: 10.1172/JCI183745
Activating antiviral immune responses potentiates immune checkpoint inhibition in glioblastoma models
Abstract
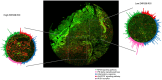
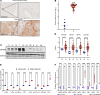
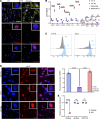
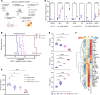
Viral mimicry refers to the activation of innate antiviral immune responses due to the induction of endogenous retroelements (REs). Viral mimicry augments antitumor immune responses and sensitizes solid tumors to immunotherapy. Here, we found that targeting what we believe to be a novel, master epigenetic regulator, Zinc Finger Protein 638 (ZNF638), induces viral mimicry in glioblastoma (GBM) preclinical models and potentiates immune checkpoint inhibition (ICI). ZNF638 recruits the HUSH complex, which precipitates repressive H3K9me3 marks on endogenous REs. In GBM, ZNF638 is associated with marked locoregional immunosuppressive transcriptional signatures, reduced endogenous RE expression, and poor immune cell infiltration. Targeting ZNF638 decreased H3K9 trimethylation, increased REs, and activated intracellular dsRNA signaling cascades. Furthermore, ZNF638 knockdown upregulated antiviral immune programs and significantly increased PD-L1 immune checkpoint expression in diverse GBM models. Importantly, targeting ZNF638 sensitized mice to ICI in syngeneic murine orthotopic models through innate IFN signaling. This response was recapitulated in recurrent GBM (rGBM) samples with radiographic responses to checkpoint inhibition with widely increased expression of dsRNA, PD-L1, and perivascular CD8 cell infiltration, suggesting that dsRNA signaling may mediate response to immunotherapy. Finally, low ZNF638 expression was a biomarker of clinical response to ICI and improved survival in patients with rGBM and patients with melanoma. Our findings suggest that ZNF638 could serve as a target to potentiate immunotherapy in gliomas.
Keywords: Brain cancer; Cancer immunotherapy; Epigenetics; Oncology; Virology.
Figures





Update of
-
Targeting ZNF638 activates antiviral immune responses and potentiates immune checkpoint inhibition in glioblastoma.bioRxiv [Preprint]. 2024 Oct 15:2024.10.13.618076. doi: 10.1101/2024.10.13.618076. bioRxiv. 2024. Update in: J Clin Invest. 2025 Mar 17;135(6):e183745. doi: 10.1172/JCI183745. PMID: 39464150 Free PMC article. Updated. Preprint.
References
-
- Chen R, et al. Endogenous retroelements and the viral mimicry response in cancer therapy and cellular homeostasis. Cancer Discov. 2021;11(11):2707–2725. doi: 10.1158/2159-8290.CD-21-0506. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

