Uncovering atrophy progression pattern and mechanisms in individuals at risk of Alzheimer's disease
- PMID: 40092368
- PMCID: PMC11906971
- DOI: 10.1093/braincomms/fcaf099
Uncovering atrophy progression pattern and mechanisms in individuals at risk of Alzheimer's disease
Abstract
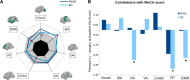
Alzheimer's disease is associated with pre-symptomatic changes in brain morphometry and accumulation of abnormal tau and amyloid-beta pathology. Studying the development of brain changes prior to symptoms onset may lead to early diagnostic biomarkers and a better understanding of Alzheimer's disease pathophysiology. Alzheimer's disease pathology is thought to arise from a combination of protein accumulation and spreading via neural connections, but how these processes influence brain atrophy progression in the pre-symptomatic phases remains unclear. Individuals with a family history of Alzheimer's disease (FHAD) have an elevated risk of Alzheimer's disease, providing an opportunity to study the pre-symptomatic phase. Here, we used structural MRI from three databases (Alzheimer's Disease Neuroimaging Initiative, Pre-symptomatic Evaluation of Experimental or Novel Treatments for Alzheimer Disease and Montreal Adult Lifespan Study) to map atrophy progression in FHAD and Alzheimer's disease and assess the constraining effects of structural connectivity on atrophy progression. Cross-sectional and longitudinal data up to 4 years were used to perform atrophy progression analysis in FHAD and Alzheimer's disease compared with controls. PET radiotracers were also used to quantify the distribution of abnormal tau and amyloid-beta protein isoforms at baseline. We first derived cortical atrophy progression maps using deformation-based morphometry from 153 FHAD, 156 Alzheimer's disease and 116 controls with similar age, education and sex at baseline. We next examined the spatial relationship between atrophy progression and spatial patterns of tau aggregates and amyloid-beta plaques deposition, structural connectivity and neurotransmitter receptor and transporter distributions. Our results show that there were similar patterns of atrophy progression in FHAD and Alzheimer's disease, notably in the cingulate, temporal and parietal cortices, with more widespread and severe atrophy in Alzheimer's disease. Both tau and amyloid-beta pathology tended to accumulate in regions that were structurally connected in FHAD and Alzheimer's disease. The pattern of atrophy and its progression also aligned with existing structural connectivity in FHAD. In Alzheimer's disease, our findings suggest that atrophy progression results from pathology propagation that occurred earlier, on a previously intact connectome. Moreover, a relationship was found between serotonin receptor spatial distribution and atrophy progression in Alzheimer's disease. The current study demonstrates that regions showing atrophy progression in FHAD and Alzheimer's disease present with specific connectivity and cellular characteristics, uncovering some of the mechanisms involved in pre-clinical and clinical neurodegeneration.
Keywords: Alzheimer’s disease; brain atrophy; protein propagation; serotonin receptor; structural connectivity.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures






References
-
- Van Duijn C, Clayton D, Chandra V, et al. Familial aggregation of Alzheimer’s disease and related disorders: A collaborative re-analysis of case-control studies. Int J Epidemiol. 1991;20(Suppl 2):S13–S20. - PubMed
-
- Borenstein A, Copenhaver C, Mortimer J. Early-life risk factors for Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20(1):63–72. - PubMed
LinkOut - more resources
Full Text Sources
